Now You Know Salt and Alkali Free Reactive Dyeing on Cotton Fabric (Part-2)
Tuesday, 22 January 2019
Edit
Salt and Alkali Free Reactive Dyeing on Cotton Fabric (Part-2)
Polyacrylamide:
Polyacrylamide (IUPAC poly (2-propenamide) or poly (1-carbamoylethylene)) is a polymer (-CH2CHCONH2-) formed from acrylamide subunits. It can be synthesized as a simple linear-chain structure or cross-linked, typically using NHYPERLINK. Polyacrylamide is not toxic. In the cross-linked form, the possibility of the monomer being present is reduced even further. It is highly water-absorbent, forming a soft gel when hydrated, used in such applications as polyacrylamide gel electrophoresis and in manufacturing soft contact lenses. In the straight-chain form, it is also used as a thickener and suspending agent. More recently, it has been used as sub dermal filler for aesthetic facial surgery.
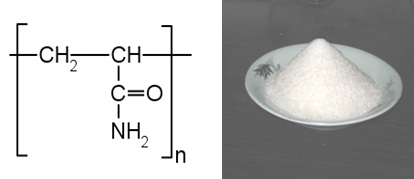 |
| Polyacrylamide |
One of the largest uses for polyacrylamide is to flocculate solids in a liquid. This process applies to water treatment, and processes like paper making. Polyacrylamide can be supplied in a powder or liquid form, with the liquid form being subcategorized as solution and emulsion polymer. Even though these products are often called 'polyacrylamide', many are actually copolymers of acryl amide and one or more other chemical species, such as an acrylic acid or a salt thereof. The main consequence of this is to give the 'modified' polymer a particular ionic character.
Another common use of polyacrylamide and its derivatives is in subsurface applications such as Enhanced Oil Recovery. High viscosity aqueous solutions can be generated with low concentrations of polyacrylamide polymers, and these can be injected to improve the economics of conventional water flooding.
It has also been advertised as a soil conditioner called Krilium by Monsanto Company in the 1950s and today "MP", which is stated to be a "unique formulation of PAM (water-soluble polyacrylamide)". It is often used for horticultural and agricultural use under trade names such as Broadleaf P4, Swell-Gel and so on. The anionic form of cross-linked polyacrylamide is frequently used as a soil conditioner on farm land and construction sites for erosion control, in order to protect the water quality of nearby rivers and streams.
The polymer is also used to make Grow-Beast toys, which expand when placed in water, such as the Test Tube Aliens. Similarly, the absorbent properties of one of its copolymers can be utilized as an additive in body-powder.
The ionic form of polyacrylamide has found an important role in the potable water treatment industry. Trivalent metal salts like ferric chloride and aluminum chloride are bridged by the long polymer chains of polyacrylamide. This results in significant enhancement of the flocculation rate. This allows water treatment plants to greatly improve the removal of total organic content (TOC) from raw water.
Environmental effects of Polyacrylamide:
Concerns have been raised that polyacrylamide used in agriculture may contaminate food with acrylamide. While polyacrylamide itself is relatively non-toxic, it is known that commercially available polyacrylamide contains minute residual amounts of acrylamide remaining from its production, usually less than 0.05% w/w.
Additionally, there are concerns that polyacrylamide may de-polymerise to form acrylamide. In a study conducted in 2003 at the Central Science Laboratory in Sand Hutton, England, polyacrylamide was treated similarly as food during cooking. It was shown that these conditions do not cause polyacrylamide to de-polymerise significantly. California requires (current as of 2010) products containing acrylamide as an ingredient to be labeled with a statement that it is "a chemical known to the State of California to cause cancer."
In a study conducted in 1997 at Kansas State University, the effect of environmental conditions on polyacrylamide were tested, and it was shown that degradation of polyacrylamide under certain conditions does in fact cause the release of acrylamide. The experimental designs of this study as well as its results and their interpretation have been questioned, and a 1999 study by the Nalco Chemical Company did not replicate the results.
Raw Materials:
- Grey fabric
- Chemicals
- Dyes
100% cotton knitted fabric (single jersey)
- Yarn count : 32Ne
- Wales per inch (WPI) : 42
- Course per inch(CPI) : 54
- Twist per inch (TPI) : 14
- Gram per square meter (GSM) : 171
- For Scouring and bleaching: (Ant creasing agent, Detergent, Sequestering agent, Caustic Soda, Soda ash, Hydrogen peroxide)
- For Neutralization and peroxide kill: (Acetic Acid, Hydrogen Peroxide Killer)
- The scoured and bleached fabric is treated with Polyacrylamide.
- For Dyeing: (Wetting agent, Sequestering agent, Reactive Dye).
- Reactive Dyes (Remazol Blue RR)
- Beaker
- Spirit lamp
- Stand
- Pipette
- pH scale
- Stop Watch
- Glass rod
- Thermometer
- Padding Machine
- Curing Machine
- Fire Box
3.1) Method:
The dye bath is set with calculated amount of dye solution and water using MLR 1:20. Enter the wetted well bleached fabric into the bath, raise the temperature to 40o c and work for 10 min then add the calculated quantity of salt in three portions at regular intervals (10 min). Raise the temperature to 50 o c and continue dyeing for 30 min, add the calculated quantity of soda ash and continue the dyeing for further 30 min. Finally take the material out, wash the material with cold water and then give soaping treatment to remove the unfixed dyestuffs and chemicals. The dyeing temperature and recipe of various reactive dyes are shown in table 3.
Fabric modification technique:
Treatment with polyacrylamide:
Pad the material with calculated quantity of polyacrylamide and water with 70% expression. After padding the material is dried at ambient temperature and then cured at 120oc for 7 min.
Dyeing of pretreated fabric:
Set the bath with calculated amount of dye solution and water. Enter the pretreated fabric into the bath. Raise the temperature to a specified level at 1.5o c / min, and dyeing continue at the set temperature for the further 60 min. Finally take out the material, soaped thoroughly and washed with cold water and dried.
3.3) Results:
Determination of fastness to washing:
The wash fastness was tested following ISO (standards) test no: 2
Discussion:
The probable mechanism for the fixation of reactive dye on the polymer treated cotton sustain may be expressed as follows:
The pretreatment of cotton fabric with polyacrylamide demonstrates the introduction of functional amino groups which increase the substantivity and also the reactivity of cotton. The cationic charged amino groups may be involved in the adsorption of anionic chromophore of reactive dyes.
The improved dye ability is postulated due to the presence of amide groups (-CONH2) available from the polyacrylamide which also tents to improve the reactivity of cellulosic substrate. The attachment of the dye molecules onto the partially-modified cellulosic substrate is by covalent bonding since no dyes strips out from the dyed sample. This is also indicative through the fastness properties washfastness.
The fastness values (given in table 1 and 2) of all such dyed samples are quite satisfactory and comparable with those of conventional dyed samples. The dry crease recovery angle values of the polymer treated samples are 80o while that of conventional dyed sample is 68o. Therefore, as expected, the polymer treated dyed samples indicate an improvement in the wrinkle recovery.
A high level of dye exhaustion on the treated fabric can be achieve in the absence of salt and alkali at a temperature as low as (Normally at 60-80oc) that used in the conventional dyeing process. Further increases in temperature may improve dye bath exhaustion, but only to a limited extent. However higher temperatures (90-100oc) are generally recommended for dyeing modified fabrics to obtain better penetration and fixation.
Modern Method
Recipe:
For scouring and bleaching:
 |
| Table: 03 |
Treatment with polyacrylamide:
Pad the material with calculated quantity of polyacrylamide and water with 70% expression. After padding the material is dried at ambient temperature and then cured at 120oc for 7 min.
Dyeing of pretreated fabric:
Set the bath with calculated amount of dye solution and water. Enter the pretreated fabric into the bath. Raise the temperature to a specified level at 1.5o c / min, and dyeing continue at the set temperature for the further 60 min. Finally take out the material, soaped thoroughly and washed with cold water and dried.
3.3) Results:
Determination of fastness to washing:
The wash fastness was tested following ISO (standards) test no: 2
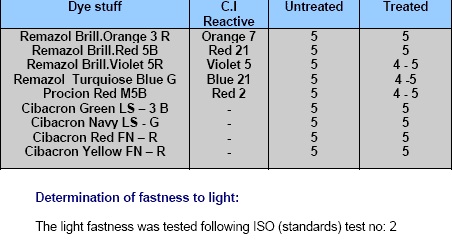 |
| Table: 04 |
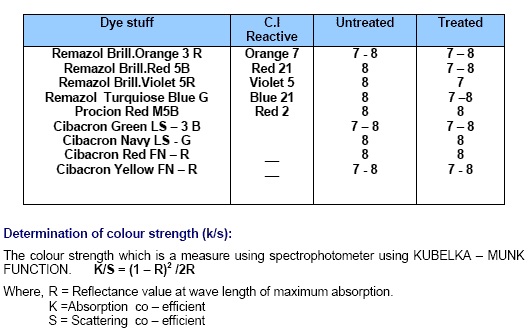 |
| Table: 05 |
 |
| Table: 06 |
The probable mechanism for the fixation of reactive dye on the polymer treated cotton sustain may be expressed as follows:
The pretreatment of cotton fabric with polyacrylamide demonstrates the introduction of functional amino groups which increase the substantivity and also the reactivity of cotton. The cationic charged amino groups may be involved in the adsorption of anionic chromophore of reactive dyes.
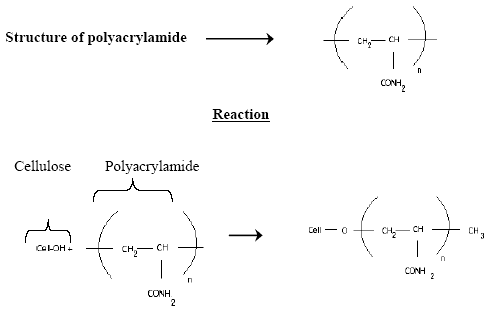 |
| Fig: 03 |
The fastness values (given in table 1 and 2) of all such dyed samples are quite satisfactory and comparable with those of conventional dyed samples. The dry crease recovery angle values of the polymer treated samples are 80o while that of conventional dyed sample is 68o. Therefore, as expected, the polymer treated dyed samples indicate an improvement in the wrinkle recovery.
A high level of dye exhaustion on the treated fabric can be achieve in the absence of salt and alkali at a temperature as low as (Normally at 60-80oc) that used in the conventional dyeing process. Further increases in temperature may improve dye bath exhaustion, but only to a limited extent. However higher temperatures (90-100oc) are generally recommended for dyeing modified fabrics to obtain better penetration and fixation.
Modern Method
Recipe:
For scouring and bleaching:
- Wetting agent: 0.7 gm/l
- Anti Creasing agent: 0.5 gm/l
- Sequestering agent: 0.7 gm/l
- Soda ash: 2.0 gm/l
- Hydrogen Peroxide: 3.0 gm/l
- Stabilizer: 0.7 gm/l
- M:L: 1:10
- Temp: 98° C
- Time: 60 min
- Acetic acid: 0.50 gm/l
- Peroxide Killer: 0.20 gm/l
Cotton fabric is padded with the polyacrylamide.
The fabric sample was desized using the acid desizing method. The fabric was scoured by the alkali method using a standard procedure. Then, it was subjected to a bleaching process using hydrogen peroxide as the bleaching agent.
Pretreatment:
The padding method was used for pretreatment of cotton with PVAmHCl. The pH of the pretreatment solution was maintained by the buffer potassium dihydrogen phosphate (7 gpl) and sodium hydroxide (1.45 gpl). Padding was carried out using two dips (4 min each) and two nips. Fabric samples were predried at room temperature and then baked at 102oC for 12 min in a rapid baker. Padding was done at different concentrations of PVAmHCl.
Dyeing:
The fabric was dyed with reactive dye using the procedure recommended by the dye manufacturer. One fabric sample was considered as a control sample. Exhaust dyeing was carried out at a liquor ratio of 1:30. Dyeing of the fabric pretreated with different concentrations of PVAmHCl was carried out at 80oC for 60 min. Fixation was conducted for 20 min using 6 to 8 gpl of Na2CO3 and 0.01 to 0.5 gpl of caustic lye.
Testing
The details of various tests conducted on the fabric are as follows. Colour strength (K/S Value) Colour strength K/S was measured on a Minolta Spectrophotometer. These values are calculated using the following “KUBELKA-MUNK” equation: where K is the absorption co-efficient, R is the reflectance of the dyed sample and S is the scattering co-efficient at the wavelength of maximum absorption.
Result of fastness test:
The results of wash and rubbing fastness are presented in Table 7.
The fastness properties of dyed cotton fabrics pretreated with PVAmHCl were determined. The results were compared with those of conventional dyeing. The wash fastness was excellent for all samples from the salt-free dyeing, confirming the effectiveness of dye fixation due to pretreatment with PVAmHCl. Rubbing fastness was also observed to be good when compared with that obtained by conventional dyeing.
Determination of fastness to washing:
The wash fastness was tested following ISO-105 X 63320. Here the tested temperature was 60° C which treated at 60 min. The fastness result is good.
Determination of fastness to rubbing:
The rubbing fastness was tested following ISO-105 X 12. The fastness result is good.
When the cotton fabric is treated with polyacrylamide, the primary hydroxyl groups of cellulose is partially modified into amide groups, which intern leads the cellulose to act like as wool fiber and hence reactive dyes can be dyed on cotton at neutral pH in the absence of electrolyte and alkali. The cationic charged amino groups may be involved in the adsorption of anionic chromophore of reactive dyes and the dye molecules are attached onto the partially modified cellulosic substrates by covalent bond formation.
CONCLUSION
Pretreatment of cotton with polyacrylamide enhances the possibility of dyeing cotton at neutral pH with various commercial reactive dyes. Such pretreatment, as applied through pad – dry – cure process, brings about some chemical changes in the treated fabric.
Fastness properties are adequate and quite comparable with conventionally dyed samples. The dyeing of cotton with reactive dyes using polyacrylamide in the dye bath improves the dye ability of cellulosic fabrics with reactive dyes and reducing effluent discharge.
When dyeing the modified substrates, reactive dyes can be much more efficiently exhausted and fixed onto cellulosic fabrics under neutral conditions in the absence of salt. The modifications show an overall suitability for different reactive dyes. The modified dyeing do not suffer either from a significant drop in light fastness, wash fastness.
REFERENCES
- Sample 1: 40% polyacrylamide
- Sample 2: 50% polyacrylamide
- Sample 3: 60% polyacrylamide
- Sequestering agent: 1 gm/l
- Wetting agent: 1 gm/l
- Leveling agent: 1 gm/l
- Reactive Dye (Blue): 2%
- M:L: 1:10
- Temp: 60°C
- Time: 60 min
- Fixing agent – 1 gm/l
- Temperature - 45˚C
- Time – 10 minute
- M:L: 1:10
The fabric sample was desized using the acid desizing method. The fabric was scoured by the alkali method using a standard procedure. Then, it was subjected to a bleaching process using hydrogen peroxide as the bleaching agent.
Pretreatment:
The padding method was used for pretreatment of cotton with PVAmHCl. The pH of the pretreatment solution was maintained by the buffer potassium dihydrogen phosphate (7 gpl) and sodium hydroxide (1.45 gpl). Padding was carried out using two dips (4 min each) and two nips. Fabric samples were predried at room temperature and then baked at 102oC for 12 min in a rapid baker. Padding was done at different concentrations of PVAmHCl.
Dyeing:
The fabric was dyed with reactive dye using the procedure recommended by the dye manufacturer. One fabric sample was considered as a control sample. Exhaust dyeing was carried out at a liquor ratio of 1:30. Dyeing of the fabric pretreated with different concentrations of PVAmHCl was carried out at 80oC for 60 min. Fixation was conducted for 20 min using 6 to 8 gpl of Na2CO3 and 0.01 to 0.5 gpl of caustic lye.
Testing
The details of various tests conducted on the fabric are as follows. Colour strength (K/S Value) Colour strength K/S was measured on a Minolta Spectrophotometer. These values are calculated using the following “KUBELKA-MUNK” equation: where K is the absorption co-efficient, R is the reflectance of the dyed sample and S is the scattering co-efficient at the wavelength of maximum absorption.
| No | Property | Standards | Instrument Used |
| 01 | Wash fastness | ISO-105 x 63320 | Wash fastness tester (Lander-o- meter) |
| 02 | Rubbing fastness | ISO- 105 x 12 | Crockmeter |
Table: 07
Result of fastness test:
| Sample No | Conc. Of Polyacrylamide | Color fastness to | Result |
| 1 | 40% | Wash | Good (4-5) |
| 1 | 40% | Rubbing (dry) | Good (4-5) |
| 1 | 40% | Rubbing (wet) | Good (4-5) |
| 2 | 50% | Wash | Good (4-5) |
| 2 | 50% | Rubbing (dry) | Good (4-5) |
| 2 | 50% | Rubbing (wet) | Good (4-5) |
| 3 | 60% | Wash | Good (4-5) |
| 3 | 60% | Rubbing (dry) | Good (4-5) |
| 3 | 60% | Rubbing (wet) | Good (4-5) |
Table: 08
The results of wash and rubbing fastness are presented in Table 7.
The fastness properties of dyed cotton fabrics pretreated with PVAmHCl were determined. The results were compared with those of conventional dyeing. The wash fastness was excellent for all samples from the salt-free dyeing, confirming the effectiveness of dye fixation due to pretreatment with PVAmHCl. Rubbing fastness was also observed to be good when compared with that obtained by conventional dyeing.
Determination of fastness to washing:
The wash fastness was tested following ISO-105 X 63320. Here the tested temperature was 60° C which treated at 60 min. The fastness result is good.
Determination of fastness to rubbing:
The rubbing fastness was tested following ISO-105 X 12. The fastness result is good.
When the cotton fabric is treated with polyacrylamide, the primary hydroxyl groups of cellulose is partially modified into amide groups, which intern leads the cellulose to act like as wool fiber and hence reactive dyes can be dyed on cotton at neutral pH in the absence of electrolyte and alkali. The cationic charged amino groups may be involved in the adsorption of anionic chromophore of reactive dyes and the dye molecules are attached onto the partially modified cellulosic substrates by covalent bond formation.
CONCLUSION
Pretreatment of cotton with polyacrylamide enhances the possibility of dyeing cotton at neutral pH with various commercial reactive dyes. Such pretreatment, as applied through pad – dry – cure process, brings about some chemical changes in the treated fabric.
Fastness properties are adequate and quite comparable with conventionally dyed samples. The dyeing of cotton with reactive dyes using polyacrylamide in the dye bath improves the dye ability of cellulosic fabrics with reactive dyes and reducing effluent discharge.
When dyeing the modified substrates, reactive dyes can be much more efficiently exhausted and fixed onto cellulosic fabrics under neutral conditions in the absence of salt. The modifications show an overall suitability for different reactive dyes. The modified dyeing do not suffer either from a significant drop in light fastness, wash fastness.
REFERENCES
- /search?q=salt-and-alkali-free-reactive-dyeing-part-1
- http://www.fibre2fashion.com/industry-article/textile-industry-articles/salt--alkali-free-reactive/salt--alkali-free-reactive4.asp
- http://www.autexrj.com/cms/zalaczone_pliki/3_01_11.pdf
- Technology of textile processing by Dr. V. A. Shenai, part ii.
- http://en.wikipedia.org/wiki/Polyacrylamide
- Lecture Sheet, Sumon Mozumder, Daffodil International University.