Now You Know Treatment of Cotton Fiber with Chitosan for the Improvement of Exhaustion during Dyeing with Reactive Dye (Part-3)
Sunday, 3 February 2019
Edit
Treatment of Cotton Fiber with Chitosan for the Improvement of Exhaustion during Dyeing with Reactive Dye (Part-3)
Mustaque Ahammed Mamun
Department of Textile Engineering
Dhaka University of Engineering & Technology (DUET)
Cell: +8801723300703
Email: mamuntex09@gmail.com
Department of Textile Engineering
Dhaka University of Engineering & Technology (DUET)
Cell: +8801723300703
Email: mamuntex09@gmail.com
Previous Part
2.12 Chitosan
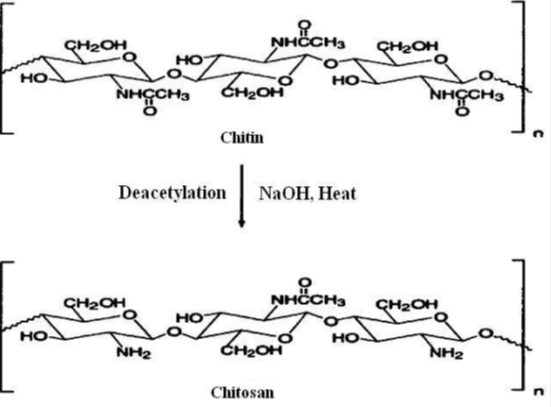
Chitin is a semicrystalline polymer with extensive inter- and intra-molecular hydrogen bonding. Hence, it is difficult to dissolve chitin in organic solvents or dilute acids under mild conditions. When chitin is deacetylated by over about 60%, it can easily be dissolved in dilute aqueous acids and is referred to as chitosan or deacetylated chitin. Thus, chitosan is the collective name given to the polymers that are deacetylated from chitin. The only difference between chitosan and chitin is the degree to which the former is deacetylated. Chitosan was first discovered in 1859 by Rouget while boiling chitin with concentrated potassium hydroxide solution that resulted in the deacetylation of chitin . In 1934, Rigby obtained two patents, one for chitosan production from chitin and other for making films and fibres from chitosan. Clark and Smith reported the first X-ray pattern of chitosan fibre. The first review on chitin and chitosan was published by Muzzarelli in 1977 . Due to its versatile physical and chemical properties, unique cationic nature, biodegradability, biocompatibility, non-toxicity and antimicrobial activity, chitosan has been extensively investigated for wide range of applications for the last three decades. Chitosan is seen as a new functional material with high application potential in various fields. Chitosan has been found to have applications in areas like food processing, cosmetics, biotechnology, agriculture, fibre formation, pharmaceuticals, medical applications, wastewater treatment, paper production, ink jet printing.
2.13 Production of Chitosan:
Chitosan is a high molecular weight and a linear polymerthat is composed of ß-1,4 linked glucosamine (GlcN) with various quantities of N-acetylated GlcN residues, Its is normally obtained by the alkaline deacetylation of chitin extracted from on a boundant source of shell fish exoskeletons.The purpose of deacetylation is to produce chitosan that is readily soluble in dilute acetic acid. It can be seen that chitin is mostly comprised of acetamide groups while chitosan is a copolymer containing acetamide and primary amino groups.
2.14 Simplified chitosan production scheme:
2.15 Physicochemical Characteristics of Chitosan
I. Degree of Deacetylation (DD)
Depending on the production method and species used, the degree of deacetylation ranges from 56% to 99%. For good solubility, the degree of deacetylation should be at least 85%. The degree of deacetylation can be obtained directly by determining amino group content of a chitosan sample or indirectly by determining acetyl content (degree of Nacetylation).
II. Molecular Weight (MW)
The MW of chitin and chitosan varies with the sources and the methods of preparation. Application of chitosan as a textile finish has shown to increase the stiffness of the fabrics, thereby affecting the feel and handle. The effect of MW on the film forming ability of chitosan and on the handle of textile substrates especially cotton will be assessed.The MW of chitosan (160.9)n.
III. Viscosity
Viscosity of chitosan solution is another property that determines its commercial applications and is affected by the degree of deacetylation, molecular weight, concentration, ionic strength, pH, and temperature . The viscosity of chitosan increases with an increase in molecular weight and concentration of chitosan, while it increases with decrease in pH in acetic acid and decreases with decreasing pH in HCl.
IV. Solubility
Water-soluble chitin can be prepared by either homogeneous deacetylation of chitin or homogeneous Nacetylation of chitosan. Water solubility is obtained by homogeneous reaction instead of heterogeneous reaction and only when the DD of chitin is about 0.5. The water-solubility was attributed to the enhanced hydrophilicity due to random distribution of acetyl groups and the destruction of the tightcrystalline structure of chitin . The solubility of chitosan is very important for its commercial applications as a textile finish, fibre or film former and for its chemical modification. Chitosan dissolves in dilute organic and mineral acids by protonation of free amino groups below pH 6.5. Acetic acid is the most preferred solvent for research and applications of chitosan. Generally, the solubility of chitosan and chitin decreases with increasing MW.
V. Colour
The colour of chitin and chitosan is associated with the carotenoid pigment whose main component is astaxanthin. The carotenoids are strongly bound with proteins in the epithelial layer of the exoskeleton of chitin. The carotenoid level in crustacean is very low and varies depending on dietary pigment availability, crustacean size, its maturation, and genetic difference.
2.16 Chemical Properties of Chitosan
The chemical properties of chitosan are as follows:
The biological properties of chitosan are as follows:
a) Biocompatible
3.1. Materials
The raw fabric is collected from local market whose Specification are given below.
 b) Dye
b) Dye
The dye which we used of thesis purpose the Specification of this Dye are given below.
Chitin is a semicrystalline polymer with extensive inter- and intra-molecular hydrogen bonding. Hence, it is difficult to dissolve chitin in organic solvents or dilute acids under mild conditions. When chitin is deacetylated by over about 60%, it can easily be dissolved in dilute aqueous acids and is referred to as chitosan or deacetylated chitin. Thus, chitosan is the collective name given to the polymers that are deacetylated from chitin. The only difference between chitosan and chitin is the degree to which the former is deacetylated. Chitosan was first discovered in 1859 by Rouget while boiling chitin with concentrated potassium hydroxide solution that resulted in the deacetylation of chitin . In 1934, Rigby obtained two patents, one for chitosan production from chitin and other for making films and fibres from chitosan. Clark and Smith reported the first X-ray pattern of chitosan fibre. The first review on chitin and chitosan was published by Muzzarelli in 1977 . Due to its versatile physical and chemical properties, unique cationic nature, biodegradability, biocompatibility, non-toxicity and antimicrobial activity, chitosan has been extensively investigated for wide range of applications for the last three decades. Chitosan is seen as a new functional material with high application potential in various fields. Chitosan has been found to have applications in areas like food processing, cosmetics, biotechnology, agriculture, fibre formation, pharmaceuticals, medical applications, wastewater treatment, paper production, ink jet printing.
2.13 Production of Chitosan:
Chitosan is a high molecular weight and a linear polymerthat is composed of ß-1,4 linked glucosamine (GlcN) with various quantities of N-acetylated GlcN residues, Its is normally obtained by the alkaline deacetylation of chitin extracted from on a boundant source of shell fish exoskeletons.The purpose of deacetylation is to produce chitosan that is readily soluble in dilute acetic acid. It can be seen that chitin is mostly comprised of acetamide groups while chitosan is a copolymer containing acetamide and primary amino groups.
 |
| Figure2.9 Deacetylation process |
Crustacean Shells
↓
Washing and Drying
↓
Demineralisation
↓
Grinding and Sieving
↓
Filtering, Washing and Drying
↓
Deacetylation
↓
Washing and Drying
↓
Chitosan
↓
Washing and Drying
↓
Demineralisation
↓
Grinding and Sieving
↓
Filtering, Washing and Drying
↓
Deacetylation
↓
Washing and Drying
↓
Chitosan
2.15 Physicochemical Characteristics of Chitosan
I. Degree of Deacetylation (DD)
Depending on the production method and species used, the degree of deacetylation ranges from 56% to 99%. For good solubility, the degree of deacetylation should be at least 85%. The degree of deacetylation can be obtained directly by determining amino group content of a chitosan sample or indirectly by determining acetyl content (degree of Nacetylation).
II. Molecular Weight (MW)
The MW of chitin and chitosan varies with the sources and the methods of preparation. Application of chitosan as a textile finish has shown to increase the stiffness of the fabrics, thereby affecting the feel and handle. The effect of MW on the film forming ability of chitosan and on the handle of textile substrates especially cotton will be assessed.The MW of chitosan (160.9)n.
III. Viscosity
Viscosity of chitosan solution is another property that determines its commercial applications and is affected by the degree of deacetylation, molecular weight, concentration, ionic strength, pH, and temperature . The viscosity of chitosan increases with an increase in molecular weight and concentration of chitosan, while it increases with decrease in pH in acetic acid and decreases with decreasing pH in HCl.
IV. Solubility
Water-soluble chitin can be prepared by either homogeneous deacetylation of chitin or homogeneous Nacetylation of chitosan. Water solubility is obtained by homogeneous reaction instead of heterogeneous reaction and only when the DD of chitin is about 0.5. The water-solubility was attributed to the enhanced hydrophilicity due to random distribution of acetyl groups and the destruction of the tightcrystalline structure of chitin . The solubility of chitosan is very important for its commercial applications as a textile finish, fibre or film former and for its chemical modification. Chitosan dissolves in dilute organic and mineral acids by protonation of free amino groups below pH 6.5. Acetic acid is the most preferred solvent for research and applications of chitosan. Generally, the solubility of chitosan and chitin decreases with increasing MW.
V. Colour
The colour of chitin and chitosan is associated with the carotenoid pigment whose main component is astaxanthin. The carotenoids are strongly bound with proteins in the epithelial layer of the exoskeleton of chitin. The carotenoid level in crustacean is very low and varies depending on dietary pigment availability, crustacean size, its maturation, and genetic difference.
2.16 Chemical Properties of Chitosan
The chemical properties of chitosan are as follows:
- Linear polyamine.
- Reactive amino groups.
- Reactive hydroxyl groups available.
- Chelates many transitional metal ions.
The biological properties of chitosan are as follows:
a) Biocompatible
- Natural polymer
- Biodegradable to normal body constituents.
- Safe and non toxic (the research chitinase is noteworthy in this respect).
CHAPTER 3
EXPERIMENTAL
EXPERIMENTAL
3.1. Materials
- Fabric.
- Reactive Dye.
- Chitosan
- Chemicals
The raw fabric is collected from local market whose Specification are given below.

The dye which we used of thesis purpose the Specification of this Dye are given below.
Table: 3.1 Specification of Dye
Common Name | Reactive Dye |
Trade Name | NOVACRON R RED TS-3B |
Company | Huntsman |
Country | Switzerland |
Colour | Red |
Odour | None |
c) Chitosan
The Specification of Chitosan are given below:
The Specification of Chitosan are given below:
Table: 3.2 Specification of Chitosan
Common name | Chitosan |
Chemical Name | Poly-(1-4)-2-Amino-2-deoxy-ß-D-Glucan |
Company name | ZHENGZHOU SIGMA CHEMICAL CO.LTD |
Origin | CHINA |
Molecular Formula | (C6H11NO4)n |
Molecular Weight | (160.9)n |
d) Chemicals
The Specification of all chemicals which was collected for experiment are given below:
3.2 Required Machineries
The Specification of all chemicals which was collected for experiment are given below:
Table: 3.3 Specification of Salt
Common name | Glauber's salt |
Chemical Name | Sodium sulfate decahydrate |
Molecular Formula | Na2SO4·10H2O |
Appearance | White or colorless monoclinic crystals |
Solubility | Water soluble |
Taste | Walty, bitter taste |
Table: 3.4 Specification of Soda Ash
Common name | Sodium carbonate (also known as washing soda or soda ash) |
Chemical Name | Sodium salt of carbonic acid |
Molecular Formula | Na2CO3 |
Appearance | White powder |
Characteristics | Water softener, fixation of dye |
Solubility | Water soluble |
Procedure | Salt (sodium chloride) and limestone |
Table: 3.5 Specification of Wetting Agent
Common Name | Wetting Agents |
Composition | Mixture of surface-active compounds. |
Appearance | Clear, colorless, slightly viscous liquid |
PH | 5.5 |
Specific Gravity at 200C | About 1 g/cm3 |
Suitable dilute | Very stable in hard water and to salts, alkalis, acids and bleaching agents. |
Table: 3.6 Specification of Leveling Agent
Common Name | Leveling Agents |
Chemical basis | Mixture of an addition complexing agent and polymers. |
Appearance | Coffee Color, low-viscosity liquid |
PH | About 7.0 |
Specific gravity at 20οC | About 1.1 |
Suitable dilute | Cold or warm water |
General stability | Stable in hard water, Alkalis and acids. |
3.2 Required Machineries
Table: 3.7 Machineries Used.
Name of Machine | Model | Brand | Origin |
Infra Red Lab Dyeing Machine | Supermat | Co-Power | Taiwan |
Washing and Dry Cleaning Color Fastness Tester | 415/8 | James H. Heal | UK |
Crock Master Color Fastness to Rubbing Tester | 670 Hand Driven crock Master | James H.Heal | UK |
Spectrophotometer | 650 | USA | |
Color matching cabinet |