Now You Know Treatment of Cotton Fiber with Chitosan for the Improvement of Exhaustion during Dyeing with Reactive Dye (Part-2)
Sunday, 3 February 2019
Edit
Treatment of Cotton Fiber with Chitosan for the Improvement of Exhaustion during Dyeing with Reactive Dye (Part-2)
Mustaque Ahammed Mamun
Department of Textile Engineering
Dhaka University of Engineering & Technology (DUET)
Cell: +8801723300703
Email: mamuntex09@gmail.com
Department of Textile Engineering
Dhaka University of Engineering & Technology (DUET)
Cell: +8801723300703
Email: mamuntex09@gmail.com
Previous Part
2.6 Reactive Dye
A dye, which is capable of reacting chemically with a substrate to form a covalent dye substrate linkage, is known as reactive dye. Here the dye contains a reactive group and this reactive group makes covalent bond with the fibre polymer and act as an integral part of fibre. This covalent bond is formed between the dye molecules and the terminal –OH (hydroxyl) group of cellulosic fibres on between the dye molecules and the terminal –NH2 (amino) group of polyamide or wool fibres. Reactive dyes in the simplest terms, all reactive dyes are made up of three basic units, a chromophore, a bridge and a reactive group/ groups (either a haloheterocycle or an activated double bond). One problem is that instead of reacting with the -OH groups on the cellulose, the fibre-reactive group may react with the HO- ions in the alkali solution and become hydrolysed. The hydrolysed dye cannot react further. This must be washed out of the fabric before use.
2.7 Properties of Reactive Dye:
- Reactive dyes are anionic dyes, which are used for dyeing cellulose, protein and polyamide fibres.
- Reactive dyes are found in power, liquid and print paste form.
- During dyeing the reactive group of this dye forms covalent bond with fibre polymer and becomes an integral part of the fibre.
- Reactive dyes are soluble in water.
- They have very good light fastness with rating about 6. The dyes have very stable electron arrangement and can protect the degrading effect of ultra-violet ray.
- Textile materials dyed with reactive dyes have very good wash fastness with rating Reactive dye gives brighter shades and have moderate rubbing fastness.
- Dyeing method of reactive dyes is easy. It requires less time and low temperature for dyeing.
- Reactive dyes are comparatively cheap.
- Reactive dyes have good perspiration fastness with rating 4-5.
- Reactive dyes have good perspiration fastness.
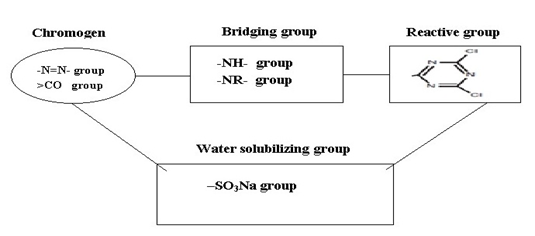 |
| Figure: 2.3 Typical Components of a Reactive Dye |
2.9 Chemical Reactions between Reactive Dyes and Fibers:
a) Nucleophilic Substitution:
Nucleophilic substitution characterizes dye-fiber fixation that occurs when a leaving group in the reactive system is displaced as a result of an interaction with a nucleophilic group on the polymer chain. Nucleophilic substitution is facilitated by the electron withdrawing properties of the aromatic nitrogens, and the chlorine, and the anionic intermediate is resonance stabilised as well. This resonance means that the negative charge is delocalised onto the electronegative nitrogens.
For example:


The major fibre-reactive group which reacts this way contains six-membered, heterocyclic, aromatic rings, with halogen substituents.
For example, the Procion dye-

The reaction of a monochlorotriazine reactive dye with a hydroxy group of cellulose is typical of this process.
a)

 |
| Figure: 2.4 Reaction of a Monochlorotriazine Dye with Cellulose |
 |
Figure: 2.5 Hydrolysis of a Monochlorotriazine Dye |
Nucleophilic addition characterizes the dye-fiber reaction in which a nucleophilic group in the fiber adds across an activated carbon-carbon double bond in the reactive group. Most of reactive systems used contain a vinylsulphone moiety. The vinylsulphone reactive group itself is usually not present in commercial form of the dyes employed. Instead, more stable precursor such as the β-sulphatoethylsulphone group is used. The two-stagenprocess associated with fiber fixation is structurally related dyes containing a β-sulphatoethylsulphamoyl group probably form a cyclic compound capable of reacting with cellulose to give cellulose ether.
Systems based on activated double bonds also undergo a competitive hydrolysis reaction.
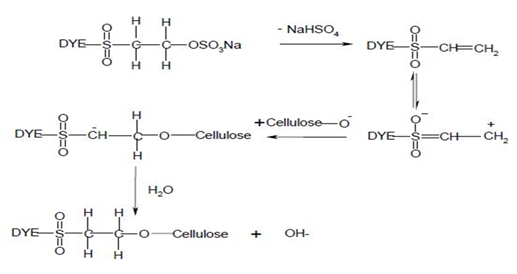 |
| Figure: 2.6 Nucleophilic Addition Involving to a Vinylsulphone Dye and Cellulose. |
 |
| Figure: 2.7 Reaction of Water with a Vinylsulphone Dye |
Chitin is the second most abundant polysaccharide on earth after cellulose. It is found in the shells of crustaceans such as crabs, shrimps and lobster, in the exoskeleton of insectsand molluscs and in the cell walls of certain fungi . Chitin was first isolated by Braconnot in 1811 and called it fungine. Later, in 1823, Odier found it in insects and named it as chitin . Chitin is mostly produced from crab and shrimp shells which are dumped as huge wastes by the seafood industry. The crustacean shell waste contains 30-50% calcium carbonate, 30-40% protein and 20-30% chitin on a dry mass basis.
2.11 Structure of Chitin, Chitosan and Cellulose
Chemically, Chitin is a homopolymer of 2-acetamido-2-deoxy-β-D-glucopyranose with some of the glucopyranose residues existing as 2-amino-2-deoxy-β-Dglucopyranose. Its molecular weight, purity, and crystal morphology are dependent on their sources . It occurs naturally as one of three crystalline polymorphic forms; α-, β- or γ-chitin differing in chain packing in crystalline regions . α-chitin has anti-parallel chains, β-chitin has a parallel stack structure while arrangement of two parallel chains and one anti-parallel chain has been suggested for γ-chitin. Both α- and β-chitins possess C=O·····H−N intermolecular hydrogen bonds, but the intermolecular hydrogen bonds between −CH2OH groups are present in the α-chitin and absent in the β-chitin . Therefore, β-chitin swells easily in water to produce hydrates unlike α-chitin, which has a strong three-dimensional hydrogen bond network. β-chitin is found in squid and marine diatoms and is rare while α-chitin is most abundantly found in crustaceans, insects, and fungi. Hence, chitosan is commercially prepared from α-chitin. The molecular structure of chitin, chitosan and cellulose are shown in Figure2.8
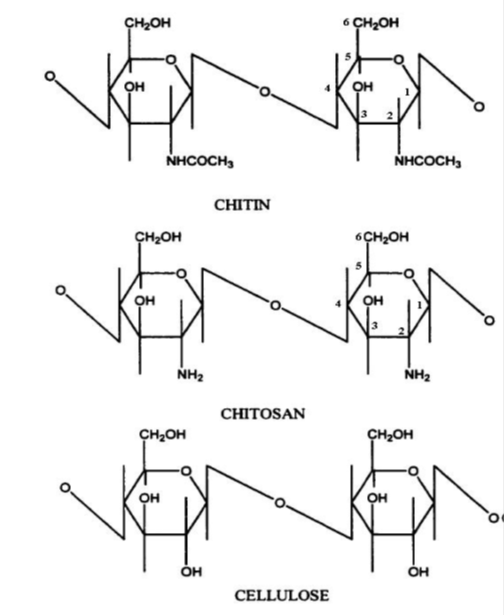 |
| Figure2.8 Molecular structure of chitin, chitosan and cellulose |