Now You Know Treatment of Cotton Fiber with Chitosan for the Improvement of Exhaustion during Dyeing with Reactive Dye
Sunday, 3 February 2019
Edit
Treatment of Cotton Fiber with Chitosan for the Improvement of Exhaustion during Dyeing with Reactive Dye
Mustaque Ahammed Mamun
Department of Textile Engineering
Dhaka University of Engineering & Technology (DUET)
Cell: +8801723300703
Email: mamuntex09@gmail.com
Department of Textile Engineering
Dhaka University of Engineering & Technology (DUET)
Cell: +8801723300703
Email: mamuntex09@gmail.com
CHAPTER 1
INTRODUCTION
INTRODUCTION
1.1 Background
Cotton fiber is the most useable fiber (E.R.trotman et al. 1975). About 90 countries in the world used cotton fiber for various purposes. This fiber is used due to some reason such as one of the first advantages of cotton is comfort. Cotton is softer so it will it feel much more comfortable to wear. The advantages of cotton include inexpensive, there is an unlimited supply. It can be worn in the summer (warm days), or winter(cool days). Cotton also absorbs water and moisture easily. The uses of it are wearing apparel, home furnishing, hygiene uses, and eventually medical uses. It is a natural fiber so it is hypo allergenic, biodegradable, easily cleaned, breathable, light and easily colored. It can be dyed by using various colors or dyestuffs (M.Lewin et al., 2010).
Cotton fiber can be dyed with various dyes such as direct dye, vat dye, sulphur dye, mordant dye, azoic dye, reactive dye. The wash fastness of direct dye is not good (2-3) (E.R.trotman et al., 1975). Rubbing fastness of vat dye is not also good. Dyeing procedure of vat dye is difficult. It is costly. Sulphur dye is unhygienic for environment. Shading is not more than 10%. Limited range of color. Bronziness and Tendering effect are common defects in sulphur dyeing. The azoic dyeing procedure is complicated and time-consuming application procedures. On the other hand reactive dyes have good fastness properties owing to the covalent bonding that occurs during dyeing (Broadbent et al. 2001).
Reactive dyeing is now the most important method for the coloration of cellulosic fibres. Cotton is made of cellulose molecules which react with the dye. During reactive dyeing the hydrogen atom in the cellulose molecule combines with the chlorine (cl2) atom in the dyeing process and results in a bond. Reactive dyes used to dye cellulosic fibres. The dyes contain a reactive group, either a haloheterocycle or an activated double bond, that, when applied to a fibre in an alkaline dye bath, forms a chemical covalent bond with a hydroxyl group on the cellulosic fibre. There are some benefits, because the reactive dye consists with chromophores of dye part, bridging groups, reactive group bearing parts and reactive groups. Reactive groups are the responsible for the fixation on alkali condition. All of the reactive dyes are organic compounds. Organic compounds are much more harmful than the others.
The main disadvantage of reactive dyes is hydrolysis of reactive dye during the application of reactive dyes to cellulose fibers under highly alkaline conditions, a competing hydrolysis reaction takes place, originating in the non-reactive oxi-dye form, which is lost for dyeing and passes into the waste water. Unfixed or hydrolyzed reactive dye has to be washed off thoroughly in order to achieve the desired superior wet fastness of the reactive dyeing. As much as 50% of the total cost 0f a reactive dyeing process must be attributed to the washing off stages treatment of the resulting effluent. This aspect of the process should be recognized a major limitation that prevents reactive dyes from achieving the degree of success that was predicted for them at the time of their discovery. This hydrolysed dye is discharged as colored effluent effluent cost is risen up. Reactive dye hydrolysates are not easily adsorbed by sewage sludge in abiological clarification plant (D Fiebig et al., 1998). Moreover color is not easily removed by effluent treatment processes and in many cases the dyes are not readily biodegradable.this unhydrolysed dyes may pose an environment hazard. So for decreasing hydrolysis salt is used but recently a compound is used before dyeing for increasing absorption which name is chitosan.
Chitosan is a non-toxic and chemically reactive, and biocompatible natural functional polymer, and has long been used as a biopolymer and natural material in many fields. Recently, chitosan effects widely studied are antistatic, bacteriostatic, biocompatibility properties conferred on various textiles (M. Sundrarajan et al. 2010). According to some previous experiences, wrinkle-resistance finishing with chitosan for fabric can improve its wrinkle degree but its handle and mechanical property got worse to a certain extent. Chitosan adsorption also increased the moisture absorption of the fibers. Chitosan with lower molecular weight increased the hydrophilicity of the treated fibers, but chitosan with higher molecular weight, decreased it.
1.2 Objectives of the thesis
The main objectives of the study to increase the dyeability reactivity dye by using eco-friendly biodegradable chitosan.
- To get desired exhaustion of dye in dyeing of cotton fabric with reactive dyes by using salt.
- To improve depth of shade cotton fabric by using chitosan.
- To improve the dye uptake and also to reduce the effluent load.
- To decrease the rate of hydrolysis of reactive dye.
- To improve the crease recovery of cotton fabric.
CHAPTER 2
LITERATURE REVIEW
LITERATURE REVIEW
2.1 Cotton Fiber
Cotton is unique in nature which has the highest percentage of cellulose. It varies from 90% to 95%. This concentration depends upon many factors, such that maturity level and variety of cotton, place on cottonseed from where it is drawn. In cotton fiber there is certain non-cellulosic matter, which plays important role in growth and on forth coming processes. These are located either on the surface of the fiber or in center of fiber means inside the lumen. Being a natural fiber, there are many factors which affect the structure of cotton cellulose. There are primary and secondary walls in the cotton cellulose. Primary wall or outer wall is less crystalline and has less percentage of cellulose. Whereas, secondary wall is around the lumen and consist of pure cellulose cotton fiber is composed of the crystalline and amorphous structure.
2.1.1 Chemical Structure of Cotton Fiber
The molecular structure of cellulose has always been of great interest to scientists and over time several structures have been proposed. The linear polymer, β-D-glucopyronose with 1,4-glycosidic bonds, is the widely accepted structure for cellulose. Consequently it may be considered as a polyhydric alcohol. Each glucopyranose ring in the cellulose chain contains three hydroxyl groups, a primary hydroxyl group in the 6- position and secondary hydroxyl groups in the 2- and 3- positions.
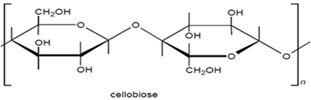 |
| Figure2.1: Cellubiose Unit. |
The basic ingredients, that are responsible for complicated interconnections in the primary wall, are cellulose, hemicelluloses, pectins, proteins and ions. These components are present throughout the primary wall. The only difference is the concentration and nature of each component, as when approaching the secondary wall. In the secondary wall, only crystalline cellulose is present, which is highly ordered and has compact structure the cellulose fibrils in the secondary wall are laying parallel to one another.
Table2.1: Typical Composition of Dry Mature Cotton Fiber
Constituents | Composition (%) | |
Whole fiber | Outer layer | |
Cellulose | 94 | 54 |
Waxes | 0.6-1.3 | 14 |
Pectin substances | 0.9-1.2 | 9 |
Protein (nitrogensubstances) | 0.6-1.3 | 8 |
Ash | 1.2 | 3 |
Organic Acid | 0.8 | - |
Others | 1.4 12 | |
2.1.3 Chemistry of Cotton Cellulose
Cellulose is an insoluble substance and mainly composed of polysaccharide, which holds chains of glucose monomers. It is only soluble in some specific solvents. It is the main constituent of plant cell walls and as well as of vegetable fibers. Cotton is one of the vegetable fibers which have the highest percentage of cellulose. There is a diverse structures and compositions of cellulose of cotton. Its structure makes it divergent from other naturally occurring matters. Cotton cellulose is unique in many ways and possesses distinct characteristics which make it highly useful for many purposes. It has been assumed that cotton cellulose structure is based on glucose unit only. However, it is also believed that there is modest amount of pentose is present, which is removed during scouring process. There is no evidence of the presence of pentose. Cotton cellulose is highly crystalline in nature and well oriented and has along and rigid molecular structure. The 1,4-D glucopyranose are the principle building blocks of cotton cellulose chain and are linked by l,4-glucodic bonds. Free rotation of the anhydrogluco-pyranose C-O-C link is stopped by steric effects. There are three hydroxyl groups attached to each anhydroglucose. One group is attached at C-6 and two at C-2 and C-3. Due to the presence of hydroxyl groups and the chain conformation, there are many more bonds possible (inter molecule and intramolecular). Such bonds make the fiber more rigid by increasing the rigidity of the structure of cotton cellulose.
2.1.4 Cross Section of Cotton Fiber
The cell wall is a dynamic structure which composition and form can change markedly, not only during cell growth but also after the cells have become matured. The cotton fiber is structurally built up into concentric zones and a hollow central core known as the lumen.
The mature fiber essentially consists of (from outside to inside) - the cuticle i.e. the outermost layer, the primary cell wall, the secondary wall and the lumen. Figure 1.1 systematically shows the different layers present in the cotton fiber with the compositions of each layer. Cotton contains nearly 90% of cellulose and around 10% of non-cellulosic substances, which are mainly located in the cuticle and primary wall of the fiber. Typical components in dry mature cotton fibers most of the non-cellulosic materials are present in the outer layers of cotton fiber.
Figure illustrates schematically the distribution of cellulose and other non-cellulosic materials in the various layers of cotton fiber. The outermost layer is the cuticle. It is a thin film of mostly fats and waxes. Owing to non-structured orientation of cellulose and non-cellulosic materials, the primary wall surfaces is unorganized and open. This gives the flexibility to the primary wall, which is required during the cell growth.
Cellulose is an insoluble substance and mainly composed of polysaccharide, which holds chains of glucose monomers. It is only soluble in some specific solvents. It is the main constituent of plant cell walls and as well as of vegetable fibers. Cotton is one of the vegetable fibers which have the highest percentage of cellulose. There is a diverse structures and compositions of cellulose of cotton. Its structure makes it divergent from other naturally occurring matters. Cotton cellulose is unique in many ways and possesses distinct characteristics which make it highly useful for many purposes. It has been assumed that cotton cellulose structure is based on glucose unit only. However, it is also believed that there is modest amount of pentose is present, which is removed during scouring process. There is no evidence of the presence of pentose. Cotton cellulose is highly crystalline in nature and well oriented and has along and rigid molecular structure. The 1,4-D glucopyranose are the principle building blocks of cotton cellulose chain and are linked by l,4-glucodic bonds. Free rotation of the anhydrogluco-pyranose C-O-C link is stopped by steric effects. There are three hydroxyl groups attached to each anhydroglucose. One group is attached at C-6 and two at C-2 and C-3. Due to the presence of hydroxyl groups and the chain conformation, there are many more bonds possible (inter molecule and intramolecular). Such bonds make the fiber more rigid by increasing the rigidity of the structure of cotton cellulose.
2.1.4 Cross Section of Cotton Fiber
The cell wall is a dynamic structure which composition and form can change markedly, not only during cell growth but also after the cells have become matured. The cotton fiber is structurally built up into concentric zones and a hollow central core known as the lumen.
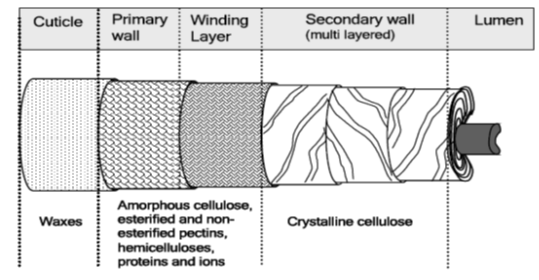 |
| Figure 2.2: Schematic representation of mature cotton fiber showing its Various layers. |
Figure illustrates schematically the distribution of cellulose and other non-cellulosic materials in the various layers of cotton fiber. The outermost layer is the cuticle. It is a thin film of mostly fats and waxes. Owing to non-structured orientation of cellulose and non-cellulosic materials, the primary wall surfaces is unorganized and open. This gives the flexibility to the primary wall, which is required during the cell growth.