Yaa Handling Of Cotton Wool Fiber Alongside Chitosan For The Improvement Of Exhaustion During Dyeing Alongside Reactive Dye (Part-2)
Wednesday, 19 December 2018
Edit
Treatment of Cotton Fiber amongst Chitosan for the Improvement of Exhaustion during Dyeing amongst Reactive Dye (Part-2)
Mustaque Ahammed Mamun
Department of Textile Engineering
Dhaka University of Engineering & Technology (DUET)
Cell: +8801723300703
Email: mamuntex09@gmail.com
Department of Textile Engineering
Dhaka University of Engineering & Technology (DUET)
Cell: +8801723300703
Email: mamuntex09@gmail.com
Previous Part
2.6 Reactive Dye
A dye, which is capable of reacting chemically amongst a substrate to shape a covalent dye substrate linkage, is known equally reactive dye. Here the dye contains a reactive grouping too this reactive grouping makes covalent bond amongst the fibre polymer too deed equally an integral purpose of fibre. This covalent bond is formed betwixt the dye molecules too the concluding –OH (hydroxyl) grouping of cellulosic fibres on betwixt the dye molecules too the concluding –NH2 (amino) grouping of polyamide or wool fibres. Reactive dyes inward the simplest terms, all reactive dyes are made upward of 3 basic units, a chromophore, a distich too a reactive group/ groups (either a haloheterocycle or an activated double bond). One work is that instead of reacting amongst the -OH groups on the cellulose, the fibre-reactive grouping may react amongst the HO- ions inward the alkali solution too locomote hydrolysed. The hydrolysed dye cannot react further. This must last washed out of the cloth earlier use.
2.7 Properties of Reactive Dye:
- Reactive dyes are anionic dyes, which are used for dyeing cellulose, poly peptide too polyamide fibres.
- Reactive dyes are works life inward power, liquid too impress glue form.
- During dyeing the reactive grouping of this dye forms covalent bond amongst fibre polymer too becomes an integral purpose of the fibre.
- Reactive dyes are soluble inward water.
- They convey really adept low-cal fastness amongst rating close 6. The dyes convey really stable electron organisation too tin protect the degrading number of ultra-violet ray.
- Textile materials dyed amongst reactive dyes convey really adept launder fastness amongst rating Reactive dye gives brighter shades too convey moderate rubbing fastness.
- Dyeing method of reactive dyes is easy. It requires less fourth dimension too frigidness for dyeing.
- Reactive dyes are comparatively cheap.
- Reactive dyes convey adept perspiration fastness amongst rating 4-5.
- Reactive dyes convey adept perspiration fastness.
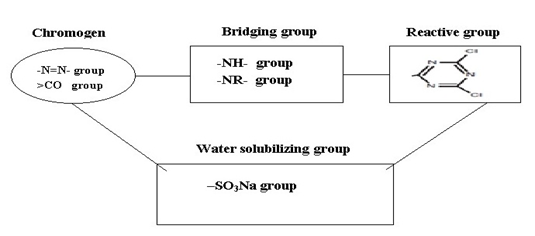 |
| Figure: 2.3 Typical Components of a Reactive Dye |
2.9 Chemical Reactions betwixt Reactive Dyes too Fibers:
a) Nucleophilic Substitution:
Nucleophilic exchange characterizes dye-fiber fixation that occurs when a leaving grouping inward the reactive scheme is displaced equally a outcome of an interaction amongst a nucleophilic grouping on the polymer chain. Nucleophilic exchange is facilitated past times the electron withdrawing properties of the aromatic nitrogens, too the chlorine, too the anionic intermediate is resonance stabilised equally well. This resonance agency that the negative accuse is delocalised onto the electronegative nitrogens.
For example:


The major fibre-reactive grouping which reacts this way contains six-membered, heterocyclic, aromatic rings, amongst element of group VII substituents.
For example, the Procion dye-

The reaction of a monochlorotriazine reactive dye amongst a hydroxy grouping of cellulose is typical of this process.
a)

 |
| Figure: 2.4 Reaction of a Monochlorotriazine Dye amongst Cellulose |
 |
Figure: 2.5 Hydrolysis of a Monochlorotriazine Dye |
Nucleophilic add-on characterizes the dye-fiber reaction inward which a nucleophilic grouping inward the fiber adds across an activated carbon-carbon double bond inward the reactive group. Most of reactive systems used comprise a vinylsulphone moiety. The vinylsulphone reactive grouping itself is ordinarily non introduce inward commercial shape of the dyes employed. Instead, to a greater extent than stable precursor such equally the β-sulphatoethylsulphone grouping is used. The two-stagenprocess associated amongst fiber fixation is structurally related dyes containing a β-sulphatoethylsulphamoyl grouping in all likelihood shape a cyclic chemical compound capable of reacting amongst cellulose to hand cellulose ether.
Systems based on activated double bonds too undergo a competitive hydrolysis reaction.
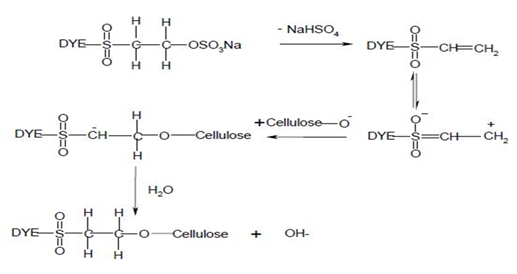 |
| Figure: 2.6 Nucleophilic Addition Involving to a Vinylsulphone Dye too Cellulose. |
 |
| Figure: 2.7 Reaction of Water amongst a Vinylsulphone Dye |
Chitin is the bit most abundant polysaccharide on public afterward cellulose. It is works life inward the shells of crustaceans such equally crabs, shrimps too lobster, inward the exoskeleton of insectsand molluscs too inward the jail cellular telephone walls of surely fungi . Chitin was get-go isolated past times Braconnot inward 1811 too called it fungine. Later, inward 1823, Odier works life it inward insects too named it equally chitin . Chitin is to a greater extent than ofttimes than non produced from crab too shrimp shells which are dumped equally huge wastes past times the seafood industry. The crustacean trounce waste matter contains 30-50% calcium carbonate, 30-40% poly peptide too 20-30% chitin on a dry out volume basis.
2.11 Structure of Chitin, Chitosan too Cellulose
Chemically, Chitin is a homopolymer of 2-acetamido-2-deoxy-β-D-glucopyranose with unopen to of the glucopyranose residues existing equally 2-amino-2-deoxy-β-Dglucopyranose. Its molecular weight, purity, too crystal morphology are subject on their sources . It occurs naturally equally i of 3 crystalline polymorphic forms; α-, β- or γ-chitin differing inward chain packing inward crystalline regions . α-chitin has anti-parallel chains, β-chitin has a parallel stack construction piece organisation of 2 parallel chains too i anti-parallel chain has been suggested for γ-chitin. Both α- too β-chitins possess C=O·····H−N intermolecular hydrogen bonds, but the intermolecular hydrogen bonds betwixt −CH2OH groups are introduce inward the α-chitin too absent inward the β-chitin . Therefore, β-chitin swells easily inward H2O to hit hydrates dissimilar α-chitin, which has a potent three-dimensional hydrogen bond network. β-chitin is works life inward squid too marine diatoms too is rare piece α-chitin is most abundantly works life inward crustaceans, insects, too fungi. Hence, chitosan is commercially prepared from α-chitin. The molecular construction of chitin, chitosan too cellulose are shown inward Figure2.8
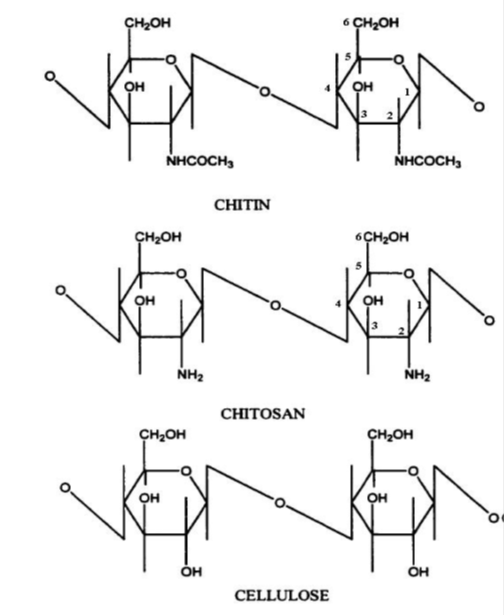 |
| Figure2.8 Molecular construction of chitin, chitosan too cellulose |