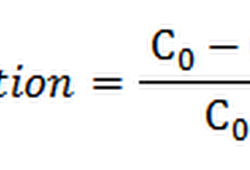Yaa Tabular Array Salt Costless Dyeing Of Cotton Fiber Cloth Amongst Reactive Dyes
Friday, 14 December 2018
Edit
Salt Free Dyeing of Cotton Fabric Using Reactive Dye
Yogesh.T
Dept of Textile Chemistry
SSM College of Engineering
Komarapalayam – 638 183, Republic of Republic of India
Email: yogeshdtp66@gmail.com
Dept of Textile Chemistry
SSM College of Engineering
Komarapalayam – 638 183, Republic of Republic of India
Email: yogeshdtp66@gmail.com
ABSTRACT
Cellulose fabrics dyed alongside reactive dyes require a large amount of tabular array salt together with alkali, which impact environmental pollution together with fresh watercourses. Due to the hydrolysis of the dye during dyeing, the dyeing effluent consists of large amount of hydrolyzed dye. And it requires a high book of H2O to take away hydrolyzed dye inward washing process. The cotton fiber cloth is dyed alongside reactive dyes using conventional method. This method requires to a greater extent than electrolytes for exhaustion together with alkali for fixation. The utilization of high tabular array salt concentration together with depression reactive dye fixation Pb to environmental work related to highly colored effluent alongside high tabular array salt content .these work tin give the axe live overcome past times improve the dye substantively of cotton fiber inward the absence of tabular array salt together with pretreating the cloth alongside polyacrylamide at different concentration together with the primary hydroxyl groups of cellulose modified into amide groups together with so handling at different concentration using pad-dry process. Pretreatment sample are dyed without tabular array salt equally an electrolyte.
The pretreated sample increases the dye uptake equally good equally deep color yield k/s value, washing together with rubbing fastness. Then original constituent of pretreatment to improve dye & fiber affinity together with likewise command the toxicity of effluent .
Samples were dyed together with evaluate the dye uptake, shade % together with likewise the discharged solution is evaluated for BOD, COD& TDS value.
CHAPTER 1
INTRODUCTION
Cotton fibers are widely applied inward textile manufacture due to its splendid properties of hygroscopicity, air permeability, biodegradability, no static electricity, goodness comfort together with this fiber has goodness strength together with it is known to render comfort, goodness wet absorption together with goodness wicking properties etc. The dyeing of these fibers are by together with large done alongside reactive dyes due to its brilliancy ,variety of hue, high wet fastness, convenient usage together with high applicability. These reactive dyes contain a reactive group, either a haloheterocycle or an activated double bond, that when applied to a fiber inward an element of group I dye bath, forms a chemic bond alongside hydroxyl grouping on the cellulosic fiber.
The popularity of reactive dyes for dyeing of cotton, environmental problems associated alongside their utilization take hold received attention. Since cotton fiber has only moderate affinity for most reactive dyes, large quantities of electrolytes such equally NaCl or Na2So4 (40-100 gpl) are usually required for exhaustion. Hence dye bathroom exhaustion together with fixation tin give the axe nonetheless live equally depression equally 50% for some dyes. Wastewater thence contains a meaning quantity of dye together with salt, leading to serious environmental problems. In recent years, reactive dyes maintain the largest annual consumption inward the Blue Planet amid the dyes used for which establishes its of import status inward the dye manufacture industry. But some problems, such equally depression dye utilization, large amount of electrolyte used together with high book of wastewater discharged, e'er be inward the application of reactive dyes. The dyeing of ane kilogram of cotton fiber alongside reactive dyes demands from lxx to 150 litre water, 0.6-0.8 Kg NaC1 together with from xxx to threescore g dyestuffs. Due to these problems this shape of dyes is the most unfavorable ane from the ecological dot of view, these effluents produced gives high values of BOD/COD (Biological oxygen demand / Chemical oxygen demand) together with increases salinity of the rivers affects the fragile biochemistry of aquatic life. More than 80,000 tons of reactive dyes are produced together with consumed each year, making it possible to quantify the full amount of pollution caused past times their use.
It has been found that pretreatment of cotton earlier dyeing tin give the axe offering a uncomplicated together with effective method of improving dye-fibre affinity, avoiding the demand for tabular array salt equally an electrolyte inward the dye bath. It has been found that polyacrylamide is a physical modifying agent.
In this physical care for a novel fiber modification technique based on cationic acrylic copolymer is retreated alongside cotton fiber fiber because it believed that pre-treated of cellulosic fiber alongside Polymer to offering an chance for increasing both the substantivity together with reactivity of fibers towards reactive dyes nether neutral conditions. The nature of a reactive polymer resin is such that it may react alongside nucleophilic sites inward cellulosic fibers or inward the polymer itself, thus fixing the polymer to the substrate. During subsequent dyeing, farther reactions betwixt the polymer together with the dyestuff, the fiber together with the dyestuff, together with the fibre together with the polymer together with tin give the axe live expected to take hold place, forming cross-link within the fibers.
The pretreatment of cotton fiber cloth alongside polyacrylamide demonstrates the introduction of functional amino groups which increment the substantivity together with likewise the reactivity of cotton. The cationic charged amino groups may live involved inward the adsorption of anionic chromophore of reactive dyes. The improved dye mightiness is postulated due to the presence of amide groups (-CONH2) available from the polyacrylamide which likewise tents to improve the reactivity of cellulosic substrate. The physical care for involve inward pad-dry physical care for at 80*c. The attachment of the dye molecules onto the partially-modified cellulosic substrate is past times covalent bonding since no dyes strips out from the dyed sample. The fastness values of all such dyed samples are quite satisfactory together with comparable alongside those of conventional dyed samples.
The popularity of reactive dyes for dyeing of cotton, environmental problems associated alongside their utilization take hold received attention. Since cotton fiber has only moderate affinity for most reactive dyes, large quantities of electrolytes such equally NaCl or Na2So4 (40-100 gpl) are usually required for exhaustion. Hence dye bathroom exhaustion together with fixation tin give the axe nonetheless live equally depression equally 50% for some dyes. Wastewater thence contains a meaning quantity of dye together with salt, leading to serious environmental problems. In recent years, reactive dyes maintain the largest annual consumption inward the Blue Planet amid the dyes used for which establishes its of import status inward the dye manufacture industry. But some problems, such equally depression dye utilization, large amount of electrolyte used together with high book of wastewater discharged, e'er be inward the application of reactive dyes. The dyeing of ane kilogram of cotton fiber alongside reactive dyes demands from lxx to 150 litre water, 0.6-0.8 Kg NaC1 together with from xxx to threescore g dyestuffs. Due to these problems this shape of dyes is the most unfavorable ane from the ecological dot of view, these effluents produced gives high values of BOD/COD (Biological oxygen demand / Chemical oxygen demand) together with increases salinity of the rivers affects the fragile biochemistry of aquatic life. More than 80,000 tons of reactive dyes are produced together with consumed each year, making it possible to quantify the full amount of pollution caused past times their use.
It has been found that pretreatment of cotton earlier dyeing tin give the axe offering a uncomplicated together with effective method of improving dye-fibre affinity, avoiding the demand for tabular array salt equally an electrolyte inward the dye bath. It has been found that polyacrylamide is a physical modifying agent.
In this physical care for a novel fiber modification technique based on cationic acrylic copolymer is retreated alongside cotton fiber fiber because it believed that pre-treated of cellulosic fiber alongside Polymer to offering an chance for increasing both the substantivity together with reactivity of fibers towards reactive dyes nether neutral conditions. The nature of a reactive polymer resin is such that it may react alongside nucleophilic sites inward cellulosic fibers or inward the polymer itself, thus fixing the polymer to the substrate. During subsequent dyeing, farther reactions betwixt the polymer together with the dyestuff, the fiber together with the dyestuff, together with the fibre together with the polymer together with tin give the axe live expected to take hold place, forming cross-link within the fibers.
The pretreatment of cotton fiber cloth alongside polyacrylamide demonstrates the introduction of functional amino groups which increment the substantivity together with likewise the reactivity of cotton. The cationic charged amino groups may live involved inward the adsorption of anionic chromophore of reactive dyes. The improved dye mightiness is postulated due to the presence of amide groups (-CONH2) available from the polyacrylamide which likewise tents to improve the reactivity of cellulosic substrate. The physical care for involve inward pad-dry physical care for at 80*c. The attachment of the dye molecules onto the partially-modified cellulosic substrate is past times covalent bonding since no dyes strips out from the dyed sample. The fastness values of all such dyed samples are quite satisfactory together with comparable alongside those of conventional dyed samples.
1.1 OBJECTIVES
- To elimination of tabular array salt equally an electrolyte.
- To cut down the hydrolysis of dye molecules.
- To accomplish maximum dye fixation.
- To improve the highest dye up-take
- To cut down depression book of H2O requirement during washing physical care for
- To improve fastness properties together with compare different fastness.
- To command depression bird of BOD,COD value
- To command depression bird of TDS value
- To cut down the environmental pollution.
CHAPTER 2
LITERATURE REVIEW
2.1 THEORIES OF COLOUR AND CONSTITUTION
A chemical compound appears colored if it selectively absorbs calorie-free inward the visible part together with reflects the calorie-free of moving ridge length inward the relaxation of the visible region. The amount of calorie-free liberate energy absorbed inward the visible spectrum is the only responsible factor for the shade of the color. The original constituent of absorbed liberate energy is to heighten the molecule from the soil state liberate energy to the excited state.
If the electrons of a molecule are tightly jump equally inward saturated chemical compound no calorie-free of visible volition live absorbed together with hence the chemical compound appears colorless. If the electrons of a molecule are loosely jump equally inward saturated chemical compound absorbance occurs inward the visible part together with hence the chemical compound appears colored.
Types together with definition
Coloring materials are mainly of 3 types. Dyes, pigments together with lakes (ingrain dyes). Influenza A virus subtype H5N1 dye has 3 parts inward its construction chromophore, chromogen together with auxochrome together with is soluble inward a specific medium nether sure enough conditions.

 Chromopores: Chromopores are grouping of atoms , the II electrons of which may larn transfer from soil state to excited state past times absorption of radiations ,thus producing the hue.
Chromopores: Chromopores are grouping of atoms , the II electrons of which may larn transfer from soil state to excited state past times absorption of radiations ,thus producing the hue. Auxochromopores: Auxochromopores are grouping of atoms which tend to increment resonance past times interacting the unshared twosome of electrons on nitrogen or oxygen atoms of the auxochromes, alongside II electrons of aromatic ring. Auxochrome is a substituted acidic or basic grouping inward dye construction to intensify depth of shade, e.g. -OH, COOH, SO.H, -NH, -NH(CH.). etc.. Further add-on of substituents to dye construction deepens the shade together with extent of deepening varies alongside increment inward molecular weight of dye.
Chromogen retains chromophore together with plays a crucial role to determine the concluding hue together with its affinity for fibre, fastness, stability, etc.
Substantivity / Affinity:
The substantivity of a dye for a fibre tin give the axe live defined equally an attraction betwixt the fibre together with the dye nether given dyeing conditions, where past times the dye is selectively extracted from the an application medium past times the fibre. The term affinity is used equally it is to a greater extent than clearly defined together with tin give the axe live given numerical value (usually inward joules per mole). It is defined equally the deviation betwixt the chemic potential of the dye inward its touchstone state inward the fibre together with the corresponding potential inward the dye. In uncomplicated terms substantivity or affinity indicates the mightiness of a dye to travel from the solution stage to the fibre.
Exhaustion:
This is a mensurate of the proportion of the dye absorbed past times the fibre relation to that remaining inward the dye bath. Thus, it indicates the amount of dye that has moved from the solution into the fibre nether given dyeing conditions. It is likewise a mensurate of the substantivity of the dye for the fibre.
Exhaustion is defined equally the volume of dye taken upward past times the stuff divided past times the full initial volume of dye inward the bath, but for a bathroom of constant volume.
For example, if the exhaustion of a dye bathroom is 75%, it agency that 75% of the dye inward the dye bathroom has moved from the dye solution or dye liquor into the fiber. The term exhaustion is mainly applicable to batch –wise dyeing which is likewise called exhaust dyeing. Textile yarn together with cloth are ofttimes dyed past times the exhaust dyeing technique.
Aggregation:
Aggregation numbers - the average number of dye molecules inward a micelle - had been determined from diffusion coefficients, electrical electrical conductivity measurements, osmotic pressures, membrane filtration, together with calorie-free scattering. Aggregation increases alongside increasing dye concentration together with decreases alongside increasing temperature. Exact aggregation numbers together with the number of incorporated counter ions, which determine the actual overall electrical accuse of the micelle, are imprecisely known. It is usually assumed that a rapid central occurs betwixt costless dye molecules inward the solution together with dye micelles of various sizes.
Material to Liquor Ratio:
This aspect refers to the weight book human relationship betwixt the fibre to live dyed together with the full book of dye bathroom .It is usually abbreviated equally MLR together with sometimes written equally M:L ratio. An M:L ratio of 1: 10 agency that a dye bathroom book of 10 litres is required to dye 1kg of dry out fibre.
The stuff to liquor ratio is likewise referred to equally an inverse ratio together with called the liquor to goods ratio or only the liquor ratio together with this ratio is given past times the next expression.
Total book of dye liquor inward ml
Liquor to goods ratio = -----------------------------------------------------------------------
Dry weight of stuff dyed inward grams.
Thus 5 gms of stuff dyed at a liquor to goods ratio of 5:1 would utilization 5x5 =25ml of dye liquor. Alternatively, 3gms of stuff dyed inward dye bathroom of 60ml has a liquor / goods ratio of 60/3 or 20 / 1 or 20:1
Shade Percentage:
Shade per centum refers to the quantity of dye taken for a dyeing expressed equally a per centum of the dry out weight of the fibre to live dyed. For example, If 1g of a dye is taken fordyeing100gof textile material, the shade per centum is referred to equally 1 % Shade. If a kilogram of fibre is required to live dyed to a3.5 5shade, the amount of dye to live taken would live (1000x 3.5 ) /100 = 35 grams.
Formula for calculation
Ml of dye solution required or added = Percentage of depth of shade x weight of fibre to live dyed inward gms.
Concentration of stock dye solution inward g/100ml
For example. If 3% shade is required to live dyed on 4gof cotton fiber yarn using a solution of ane gram of dye in200ml of H2O (ie 0.5g inward 100ml H2O ), the ml of the stock dye solution required would be, ml of dye solution required or added = 3x4 / 0.5 =24ml
2.2 THE GENERAL THEORY OF DYEING
Dyeing is the physical care for of coloring textile materials past times immersing them inward an aqueous solution of dye, called dye liquor. Normally the dye liquor consists of dye, H2O together with an auxiliary. To improve the effectiveness of dyeing, oestrus is usually applied to the dye liquor.
The full general theory of dyeing explains the interaction betwixt dye, fibre, H2O together with dye auxiliary. More specifically, it explains:
- Force of repulsion which are developed betwixt the dye molecules together with water.
- Force of attraction which are developed betwixt the dye molecules together with fibres.
The dye molecule
Dye molecules are organic molecules, which tin give the axe live classified as:
- Anionic – inward which the color is caused past times the anionic constituent of the dye molecule;
- Cationic – inward which the color is caused past times the cationic constituent of the dye molecule;
- Disperse- inward which the color is caused past times the whole molecule. The start 2 dye molecule types are applied from an aqueous solution. The 3rd is applied from an aqueous dispersion.
An essential characteristic of the dyeing physical care for is that the dye molecule must live capable of entering the fibre structure, the path for the dye molecules is provided past times the intermolecular spaces inward the fibre together with in ane lawsuit the dye has entered the fibre construction it becomes firmly attached to the surface of the molecules either past times purely physical forces (Secondary Valences) or past times chemic combination. The sometime mode of attachment is believed to live prevalent inward the dyeing of cellulosic fibres, the latter mode inward the dyeing of poly peptide fibres. Acetate rayon together with synthetic fibres resist penetration past times the dye molecules, but sure enough dyes are capable of forming a solid solution alongside the fibrous molecule; for dyeing alongside other dyes, the synthetic fibres may live swollen alongside suitable agents. Swelling of the fibers appears to play a large constituent inward dyeing of all fibres, together with is principally affected past times H2O (or solvents inward the instance of synthetics) together with past times raising the temperature of the dye bath.
The dyeing physical care for tin give the axe thus live considered equally taking house inward 3 phases:
- Attachment of the dye molecule to the surface of the fibre
- Penetration into the intermolecular spaces equally good equally diffusion through the fibre,
- Orientation (and fixation) along the long chain molecules.
Dyeing is carried out to create a sure enough ‘Shade’ past times which is meant a sure enough colour, deviation inward shade beingness due to different ‘Hue’. Influenza A virus subtype H5N1 blueish shade may, for instance take hold a greenish or a reddish hue. The amount of dye needed for the production of a sure enough depth of shade is expressed equally a per centum of the weight of the material. Influenza A virus subtype H5N1 1 % dyeing represents a shade produced past times the colouring of 100 lbs. of stuff alongside ane lb. of (commercial) dye, nether good defined dyeing conditions. It is necessary to define these weather condition because of their influence on the ‘exhaustion’ of the dye bath.
2.3 FASTNESS PROPERTIES OF DYES:
When a dye is nowadays on a cloth it is expected to take hold sure enough properties. Thus when a dyed or printed cloth (garments, drape materials, furnishing cloth etc.) is exposed to Sun calorie-free during its use, the dye should non fade or alter its colour, i.e., it should take hold high light fastness.
The dye should posse’s goodness washing fastness if the cloth dyed alongside it is used for making garments. Otherwise staining of garments which strip dyestuffs occurs during the washing of many garments. These dyes should likewise take hold goodness perspiration fastness. Perspiration of sure enough people is acidic inward nature together with of theirs, alkaline. When people wearing colored garments perspire a constituent of the dye coming into contact alongside the perspiration may live strip together with stain the undergarment or the peel of the wearer.
A unmarried dye, which dyes all the known major textile fibres is non made yet. Some of fibres are dyed alongside sure enough classes of dyes together with other fibres alongside other classes. Thus cotton, mercerized cotton, linen, viscose rayon, cupramonium rayon etc. (cellulosic fibers) are dyed alongside vat, reactive, direct, azoic (naphthol together with base), sulphur, oxidation colour, mineral color together with basic dye (after moderating). Wool together with silk (protein fibres) are dyed alongside acid dyes, basic dyes (without the utilization of mordant), acid mordant dyes (special acid dyes which tin give the axe combine alongside metals similar chromium dyeing together with create launder fast dyeing .Example metal-complex dyes (dyes containing the metallic atom inward their molecular construction so that the metallic atom demand non live incorporated into the dye during dyeing) .
Acetate together with triacetate together with polyester fibres are dyed alongside disperse dyes. Polyamide fibres are dyed alongside acid dyes, metal-complex dyes, disperse dyes. Acrylic fibres are dyed alongside basic (cationic) dyes.
Dye selection
There are numerous factors involved inward the choice of dyes for colouring a cloth inward a item shade. Some of these are:
- The types of fibres nowadays
- The shape of the textile stuff together with the grade of levelness required - bird dyeing is less critical for loose fibres, whichare afterwards blended, than it is for cloth
- The fastness properties required for whatever subsequent manufacturing processes together with for the item end-use
- The dyeing method to live used, the overall cost, together with the mechanism available
- The actual color requested past times the customer.
In 1956 Rattee + Stephen (ICI) introduced “first” reactive dyes– chlorotriazines.
Reactive dyes are chemically react alongside cotton fiber fibre together with shape covalent bond together with travel a constituent of the fibre. The reactive dyes contains several groups that are shown inward below,
- water solubilising grouping
- chromophore
- bridging grouping
- reactive grouping
- leaving grouping
- It take hold sulphonic acid groups inward the molecules together with readily soluble inward water.
- Less noun than direct dyes, hence to a greater extent than tabular array salt is required for exhaustion.
- Dyestuff react together with combine chemically (covalently) alongside cellulose, so called reactive dyes.
- Easy penetration together with goodness leveling property.
- Moderate to goodness calorie-free together with launder fastness properties.
- Formation of covalent bond occur inward element of group I medium
- These dyes, dissimilar whatever other shape of dye stuffs, react together with combine chemically (covalently) alongside cellulose together with this leading to splendid wash-fastness.
- These dyes give real vivid shades such equally orange, pink, magenta etc, which were non possible alongside other shape of dyes.
- They do non react alongside H2O nearly equally readily equally alongside cellulosic hydroxyl inward element of group I conditions, so that they tin give the axe live applied from an aqueous solution.
- Reactivity of the dyestuffs tin give the axe live reduced when desirable past times blocking ane of the reactive chlorine atoms giving H-type Procions.
- Procions are dyes alongside modest molecules; their molecules do non take hold to live real long equally those of direct dyes to fit the distance betwixt absorption sites on the fibre. Short molecules convey 2 advantages (a) Clarity together with brightness of hue together with (b) piece of cake penetration together with thence goodness leveling.
- Because in that location is some, fifty-fifty although non real much reaction betwixt procion dyestuffs together with water, it is real of import to launder the dyed fibre thoroughly build clean together with costless from the reaction production alongside water.
- The formation of the covalent bond betwixt dye together with fibre occurs nether element of group I conditions. The presence of acids may contrary this process. Perspiration together with atmospheric pollution which are both slightly acid may impact textile materials coloured alongside reactive dyes together with resultant inward some fading
- Reactive syes tin give the axe live applied to cellulosic fibres past times exhaust (batch), pad batch (sermi-continuous), continuous dyeing method.
Reactive dyes tin give the axe live classified basically into 3 groups
Group 1: Alkali Controllable
Group 2: Salt Controllable
Group 3: Temperature Controllable
On the footing of reactive system, reactive dyes tin give the axe live classified equally
Monofunctional Reactive dyes:
- Dyes are characterized past times the presence of reactive groups- ane or to a greater extent than reactive species at private locations inward the dye molecule.
- Examples of this type is mono chloro triazine, dichloro triazine together with vinylsulphone dyes
These dyes are characterized past times the presence of 2 reactive groups of same type ( MCT or DCT) or different type(MCT&VS) at 2 different locations inward the dye molecule.
Bifunctional in ane lawsuit again divided into:
1. Homo bifunctional- dyes having 2 reactive systems of same type (triazine or vinylsulphone).
2. Hetero bifunctional – dyes having 2 reactive systems of mixed type (triazine - vinylsulphone).
- These dyes take hold High exhaustion together with high fixation alongside goodness color yield.
- Less pollution
- Very pop for exhaust dyeing applications
- High exhaustion – due to high molecular weight of dyes
- High fixation – due to presence of 2 reactive systems
Reactive dyes shape a covalent bond betwixt fibre together with dye. They are classified depending on the reactive grouping nowadays together with the optimized weather condition inward which they are best used. Depending on the type of reaction, the reactive dyes are broadly divided inward to 2 categories:
A. Dyes reacting through Nucleophilic exchange reactions
B. Dyes reacting through Nucleophilic add-on reactions.
A. Dyes reacting through Nucleophilic exchange reactions:
(1) Dichlorotriazynilamino types of dyes
 |
| Figure.2.1: Structure of Dichlorotriazynilamino dyes |
(2) Monochlorotriazynylamino type of dyes
 |
| Figure 2.2: Structure of Monochlorotriazynylamino dyes |
B. Dyes reacting through Nucleophilic add-on reactions:
(1) Dyes containing Vinyl sulphone grouping
As such this is non soluble inward water, so it is marketed inward its soluble shape i.e., β - hydroxy ethylene sulphone sulphuric acid ester derivatives.
RSO2 –CH2 -CH2OSO3Na
2.4.4 Chemistry behind Reactive Dyeing:
The dyeing regulation is based on fiber reactivity together with involves the reaction of a functional grouping of the dyestuff alongside a site on the fiber to shape a covalent link betwixt the dye molecule together with the substance.
The Four structural characteristic of typical reactive dyes molecule are:
- The chromophoric grouping, contributing the color
- The reactive system, enabling the dye to react alongside hydroxy grouping inward cellulose.
- Influenza A virus subtype H5N1 bridging grouping that links the reactive arrangement to the chromophore.
- One or to a greater extent than solublising group, usually sulphuric acid substituent attached to the chromophoric grouping for their colour, although the azo chromophore –N=N- is past times itself the most important.
S------R----B----X
Where,
S = Water solubility grouping
R = Chromophore
X = Reactive System
B = Bond betwixt reactive arrangement together with Chromophore
2.5 COLD BRAND AND HOT BRAND REACTIVE DYES
The reactivity of these dyes is dyes are due to the chlorine atoms attached to the triazine ring. When 2 chlorine atoms are nowadays inward the dye molecule are called Dichloro Procions. Dichloro procions are referred to equally M-type procions together with their characteristic is that they volition combine alongside element of group I cellulose at room temperature (20oC to 30oC) together with hence they are called mutual depression temperature build reactive dyes when only ane chlorine atom is nowadays inward the dyestuff molecule, the reactivity of the dye decreases considerably together with the dyeing has to live carried out at a higher temperature (65oC to 80oC). Hence these dyes are called hot build reactive dyes. Mono chloro procions are referred to equally ‘H’ type procions. Normally ‘M’ Brands are suitable for dyeing together with H brands are suitable for printing.The instability of the solutions of the cold-dyeing procion colours was a serious disadvantage inward their application to textile printing. The stock solution for printing must live kept together with used for several hours. The reactive dyes having only ane chlorine atom inward their molecule are less reactive. Their aqueous solutions, therefore, are to a greater extent than stable together with real suitable for printing.
2.5.1 TYPES OF REACTIVE DYES:
S = Water solubility grouping
R = Chromophore
X = Reactive System
B = Bond betwixt reactive arrangement together with Chromophore
2.5 COLD BRAND AND HOT BRAND REACTIVE DYES
The reactivity of these dyes is dyes are due to the chlorine atoms attached to the triazine ring. When 2 chlorine atoms are nowadays inward the dye molecule are called Dichloro Procions. Dichloro procions are referred to equally M-type procions together with their characteristic is that they volition combine alongside element of group I cellulose at room temperature (20oC to 30oC) together with hence they are called mutual depression temperature build reactive dyes when only ane chlorine atom is nowadays inward the dyestuff molecule, the reactivity of the dye decreases considerably together with the dyeing has to live carried out at a higher temperature (65oC to 80oC). Hence these dyes are called hot build reactive dyes. Mono chloro procions are referred to equally ‘H’ type procions. Normally ‘M’ Brands are suitable for dyeing together with H brands are suitable for printing.The instability of the solutions of the cold-dyeing procion colours was a serious disadvantage inward their application to textile printing. The stock solution for printing must live kept together with used for several hours. The reactive dyes having only ane chlorine atom inward their molecule are less reactive. Their aqueous solutions, therefore, are to a greater extent than stable together with real suitable for printing.
2.5.1 TYPES OF REACTIVE DYES:
- Cold build reactive dye -‘M’ build
- Hot build reactive dye -‘H’ build (or ‘X’ brand)
- High exhaust reactive dye -‘HE’ build
- High exhaust reactive dye -‘ME’ build
- Vinyl Sulphone reactive dye - for dyeing together with printing
- Low tabular array salt reactive dye - LS dyes
MONOCHLOROTRIAZINYL, REACTIVE DYES (OR) PROCION + H BRAND
The mono chlorotriazinyl dyes or hot build reactive dyes are less reactive. Their aqueous solutions are to a greater extent than stable but they do non react alongside cellulose so readily together with the temperature of dyeing must live increased to 60oC to 70oC together with inward some cases, equally high equally 90oC to 95oC.
The dyestuffs are dissolved past times making them into a glue alongside mutual depression temperature water, followed past times dilution alongside to a greater extent than water.
 |
| Figure 2.3: Cibacron Brilliant Red B |
After treatment:
- Treat the dyed stuff alongside 1 to 2 g/l of neutral lather at boil for fifteen minutes together with launder it.
- Treat the soaped stuff alongside 2 to 3 g/l of cationic dye fixing agent at 40oC for 20 to xxx minutes together with dry out it.
Cellulosic cloth is dyed alongside reactive dyes require large amount of salt, which pollutes fresh H2O . Due to the hydrolysis of the dye, the dyeing effluent consists of large amount of hydrolyzed dye, together with it requires high book of H2O to take away the hydrolyzed dye inward washing process. The cotton fiber fabrics were dyed alongside reactive dyes using conventional method together with pre-treating the cloth alongside Polyvinylamine Chloride. Pretreated samples were dyed without using tabular array salt equally an electrolyte. It was found that pretreatment of cotton fiber fabrics alongside Polyvinylamine Chloride increases the dye uptake together with shows goodness launder fastness together with rubbing fastness. There was a slight increment inward plication recovery angle together with flexural rigidity inward pretreated sample. It is considered that Polyvinylamine Chloride is found to live effective for pretreatment inward tabular array salt costless dyeing of cotton fiber fabrics.
It has been found that pretreatment of cotton fiber earlier dyeing tin give the axe offering a uncomplicated together with effective method of improving dye-fibre affinity, avoiding the demand for tabular array salt equally electrolyte inward dye bath. It has been found that Poly (Vinylamine Chloride) [PVAmHCl] is a physical modifying agent. Its broad make of properties has found utilization inward Catalysis, Chelating, liquid chromatography, handling of wastewater, recovery of crude oil together with inward polymeric dyes.it involving chemic modification of Cellulosic. Nonreactive pretreatments including some polymers alongside affinity for cellulose tend to live desorbed during dyeing together with inhibit uptake of dye or motion it to precipitate. This study has established the value of polymeric fourth ammonium compounds, amines or amides, which may live attached to cotton fiber past times non-chemical mechanisms. Despite the encouraging results obtained alongside non-reactive polymers inward the tabular array salt costless dyeing of cotton, problems rest inward dye choice together with obtaining bird results. The aim of this piece of work to determine the effectiveness of PVAmHCl equally pretreatment agent of cotton fiber fabrics inward improving its dyeability alongside reactive dyes together with inward achieving evenness of dye uptake. It was likewise to determine the effectiveness of pretreatment of dyed fabrics on K/S value together with fastness properties similar launder fastness together with Rub fastness. Various physical properties similar tensile strength, flexural rigidity,cloth plication recovery angle together with aerial density were likewise determined to consider the number of PVAmHCl. Results obtained were analyzed to build it at some advantages of the pretreatment.
2.7.1 FUNCTION OF POLYVINYLAMINE CHLORIDE (PVAmHCl)
PVAmHCl has been used equally a physical modifying agent. Due to its broad make of properties, PVAmHCl has found utilization inward catalysis,liquid chromography, handling of wastewater, recovery of crude oil together with inward polymeric dyes. It has been used inward application equally various equally newspaper making together with biomedical research, but it’s used for modification of cotton fiber for tabular array salt costless dyeing equally non been previously reported. Interest inward PVAmHCl arises from the presence of large number of cationic sites (NH+3Cl-). Nucleophilic sites involving primary amino grouping within the PVAmHCl molecule are of item value for achieving tabular array salt costless dyeing of cotton fiber alongside reactive dyes. As the pH increases, the proportion of NH+3Cl- groups inward the molecule decreases together with that of NH2 groups increases.
Process
The cloth sample was desized past times using acid desizing method. The cloth was scoured past times alkali method using touchstone procedure. Then it was subjected to bleaching physical care for using hydrogen peroxide equally bleaching agent. Padding method was used for pretreatment of cotton fiber alongside PVAmHCl the pH of pretreatment liquor was maintained at buffer comprising potassium dihydrogen phosphate together with Sodium Hydroxide. Padding was carried out using 2 dips (4 min for each) together with 2 nips. Fabric samples were pre dried at room temperature together with and so baked at 102oC for 12 min inward rapid baker. Padding was done at different concentration of PVAmHCl.Then cotton fiber cloth dyed alongside tabular array salt together with without salt, together with so physical care for efficiency analysed together with compared.
2.8 SALT FREE DYEING WHY?
In recent years in that location has been an increasing awareness virtually environmental friendliness inward all human activities. The textile manufacture is a H2O intensive manufacture alongside H2O beingness used inward every stage of wet processing from sizing, desizing, scouring together with bleaching of fibers to the dyeing, finishing together with printing of fabrics. Every textile flora requires large volumes of H2O together with produces high volumes of effluent wastewater. The typical textile dye wastewater composition is quite complex. The demand for environmental friendly dyes together with application processes is thence real strong.
Reactive dyes take hold travel real pop for cotton fiber due to its brilliancy, diversity of hue, high wet fastness, convenient usage together with high applicability
Reactive dyes are anionic inward graphic symbol together with cotton fiber fibers likewise adopt anionic surface accuse inward H2O causing limited exhaustion of dye due to accuse repulsion. Large quantities of electrolyte (30-100 g/l) are thus added to overcome this problem. One of the major work of reactive dyeing is the large amount of electrolyte required for exhaust together with pad application [4] which leads to environmental problem. In addition, inadequate dye exhaustion together with fixation resultant inward coloured effluents. As environmental problems arising from dyeing alongside reactive dyes take hold travel critical, many studies take hold been devoted to improving the substantivity of cotton fiber fiber for reactive dyes, thus reducing or eliminating the amount of electrolyte used.
2.9 FUNCTION OF SALT IN THE DYEING PROCESS:
The tabular array salt inward the reactive dyeing increases the affinity of the dye towards the Cellulosic substrate. Salt increases the exhaustion charge per unit of measurement of reactive dyestuffs.
As reactive dyestuffs take hold a lower affinity, to a greater extent than inorganic tabular array salt is required when using reactive dyestuffs inward companionship to accelerate absorption. While the amount of inorganic tabular array salt used varies according to the type of dyestuff used, of late developed high-fixation dyestuffs alongside improved affinity allow the amount of inorganic tabular array salt to live reduced.
Due to considerations of effectiveness together with cost, both Glauber's tabular array salt together with mutual tabular array salt (sodium chloride) are used inward dyeing. In terms of their role equally an inorganic salt, these 2 are effectively the same because of the sodium cation active inward both.
2.10 ROLE OF SALT IN REACTIVE DYEING:
Inorganic salts take hold 2 original functions inward exhaustion dyeing alongside reactive dyestuffs:
- Improving the affinity of the dyestuff
- Acceleration of the dyestuff's association together with lowering of its solubility.
Reactive dye – SO3H + Na⁺ → Reactive dye SO3Na
 |
| Figure.2.4: Salt reaction |
Reactive dye – SO3Na + Water -- → Reactive dye – SO3⁻ + Na ⁺
(Dye anion) (Sodium cation)
2.11 WHY SALT IS USED IN DYEING?
The textile substrate together with dye molecule, non necessarily should take hold of homogeneous characteristics to combine alongside each other. In such case, nosotros require some catalyst to facilitate dyeing activity on fabric. Salt plays this crucial role of catalyst. Salt has an extremely high affinity for water. Broadly speaking, Salt is necessary inward 3 ways, firstly, to drive dye into textile during the dyeing physical care for inward textile. Secondly, utilization of tabular array salt leads to maximum exhaustion of dye molecules during dyeing physical care for inward textiles. Thirdly it is used equally an electrolyte for migration, adsorption together with fixation of the dyestuff to the cellulose material.
Salts plays of import role inward reactive dyeing past times improving the affinity of the dyestuff towards the fibre together with acceleration of the dyestuff's association together with lowering its solubility. Normally, Glauber's tabular array salt or mutual salt/ vacuum tabular array salt is used for this purpose. The presence of chlorine ion inward the mutual tabular array salt may motion corrosion of the equipment. Hence, Glauber's tabular array salt is e'er preferred over mutual salt. Glauber's tabular array salt is a mutual call for sodium sulfate decahydrate, Na2SO4.10H2O; it occurs equally white or colorless monoclinic crystals. Upon exposure to fairly dry out air it effloresces, forming powdery anhydrous sodium sulfate. Johann Glauber’s was the start to create the tabular array salt (from Hungarian outflow waters). Glauber's tabular array salt is H2O soluble, has a salty, bitter taste, together with is sometimes used inward medicine equally a mild laxative; it is likewise widely used inward dyeing. Vacuum tabular array salt is the mutual call of sodium chloride (NaCl).
2.12 STANDARDS NORMS FOR DISCHARGE OF EFFLUENTS WATER
S. No. | Industry | Parameter | Standard (applicable for all modes of disposal*) | |
1 | All Integrated textile units, units of Cotton / Woollen / Carpets / Polyester, Units having Printing / Dyeing / Bleaching physical care for or manufacturing and Garment units. | TREATED E | EFFLUENTS | Maximum concentration values inward mg/l except for pH, colour, and SAR |
pH | 6.5 to 8.5 | |||
Suspended Solids | 100 | |||
Colour, P.C.U Cobalt Units) | (Platinum | 150 | ||
Bio-Chemical Demand [3days (BOD3) | Oxygen at 27oC] | 30 | ||
Oil together with Grease | 10 | |||
Chemical Oxygen Demand (COD) | 250 | |||
Total Chromium equally (Cr) | 2.0 | |||
Sulphide (as S) | 2.0 | |||
Phenolic Compounds (as C6H5OH) | 1.0 | |||
Total Dissolved Solids, Inorganic (TDS) | 2100** | |||
Sodium Absorption Ratio (SAR) | 26** | |||
Ammonical Nitrogen (as N) | 50 | |||
NOTE:
- *In instance of direct disposal into rivers together with lakes, the Central Pollution Control Board (CPCB) or State Pollution Control Boards / Pollution Control Committees (SPCBs / PCCs) may specify to a greater extent than stringent standards depending upon the lineament of the recipient system.
- **Standards for TDS together with SAR shall non live applicable inward instance of marine disposal through proper marine outfall.
- The treated effluent shall live allowed to live discharged inward the ambient surroundings only after exhausting options for reuse inward industrial physical care for / irrigation inward companionship to minimise freshwater usage.
- Any textile unit of measurement attached alongside the Common Effluent Treatment Plant (CETP) shall accomplish the inlet together with treated effluent lineament standards equally specified inward series number 55 of Schedule-I to the Environment (Protection) Rules, 1986 together with shall likewise live jointly together with severally responsible for ensuring compliance.
- The standalone Micro, Small together with Medium Enterprises (MSMEs) equally per the MSME Development Act, 2006 shall run into the values specified above.
CHAPTER 3
METHODOLOGY
METHODOLOGY
 |
FABRIC:
100% cotton fiber gray evidently woven cloth was used for this project, which is having Arial density of 102 g/m2 together with it has been obtained from vetrivel textiles, erode, Republic of Republic of India together with the cloth characteristics are listed inward the Table.
Geometrical parameters of fabric:
Fabric | Ends/inch | Picks/inch | Gsm | Warp count(Ne) | Weft count(Ne) | Thickness(mm) |
100% cotton | 106 | 96 | 102 | 100 | 80 | 0.14 |
3.2 DESIZING OF COTTON FABRIC
Desizing is the physical care for of to take away size or starch from the Grey cloth which is applied during weaving together with to build the cloth to a greater extent than absorbent to facilitate dyeing together with printing.
3.2.1 Recipe:
- Enzyme - 2.0%
- Wetting agent - 1.0%
- Sodium chloride - 1.0%
- Temperature - 50-60°c
- PH -6 - vii
- Duration -1-2 Hours
The given sample is weighed past times using electronic residual
↓
Prepare the desizing bathroom laid alongside 2.0% enzyme,1.0% wetting agent together with tabular array salt past times using 1:20 stuff to liquor ratio.
↓
The temperature of the bathroom is to live raised to 50°c together with so travel inward the good wetted together with squeezed stuff into the bathroom together with worked for 2 hours
↓
Then the stuff is taken out from the bathroom together with washed thoroughly using hot H2O together with mutual depression temperature H2O
↓
Finally the stuff is squeezed together with dried.
↓
Prepare the desizing bathroom laid alongside 2.0% enzyme,1.0% wetting agent together with tabular array salt past times using 1:20 stuff to liquor ratio.
↓
The temperature of the bathroom is to live raised to 50°c together with so travel inward the good wetted together with squeezed stuff into the bathroom together with worked for 2 hours
↓
Then the stuff is taken out from the bathroom together with washed thoroughly using hot H2O together with mutual depression temperature H2O
↓
Finally the stuff is squeezed together with dried.
3.3 SCOURING OF COTTON FABRIC
Scouring is the physical care for of to take away natural impurities equally good equally added impurities.the impurities such equally oils, fats, wax together with colouring matters.the hydrophobic inward graphic symbol equally possible together with travel out the cloth inward a highly absorptive status without nether going chemic or physical harm of the material.
3.3.1 Recipe:
- Wetting agent - 1.0%
- Sodium hydroxide (NAOH) - 2-3%
- Sodium carbonate(NA2SO3) -1.0%
- Soap - 0.5%
- Temperature -boiling temperature
- PH - 10 to xi
- Duration - 2 to 3 hours
The given sample is weighed past times using electronic residual
↓
The scouring bathroom is laid alongside caustic soda 2-3%,soda ash 1%,wettind agent 1% together with lather 0.5% past times using stuff to liquor ratio equally 1:20
↓
The temperature of the bathroom is raised boil together with scouring solution is stirred
↓
The good wetted together with squeezed stuff is entered into the scoring bathroom together with is worked for 2 to 3 hours
↓
The stuff is turned out frequent intervals for proper scouring
↓
The stuff is completely immersed nether boiling liquor.the stuff should non live exposed to atmospheric air.
↓
After scouring treatment, stuff is taken out from the bathroom launder thoroughly together with so the sample is neutralized alongside 1% HCL
↓
Then launder thoroughly together with dried .thus scoured sample.
↓
The scouring bathroom is laid alongside caustic soda 2-3%,soda ash 1%,wettind agent 1% together with lather 0.5% past times using stuff to liquor ratio equally 1:20
↓
The temperature of the bathroom is raised boil together with scouring solution is stirred
↓
The good wetted together with squeezed stuff is entered into the scoring bathroom together with is worked for 2 to 3 hours
↓
The stuff is turned out frequent intervals for proper scouring
↓
The stuff is completely immersed nether boiling liquor.the stuff should non live exposed to atmospheric air.
↓
After scouring treatment, stuff is taken out from the bathroom launder thoroughly together with so the sample is neutralized alongside 1% HCL
↓
Then launder thoroughly together with dried .thus scoured sample.
3.4 BLEACHING OF COTTON
Bleaching is the physical care for of to take away the natural colouring affair together with whatever other colouring affair is removed from cotton.then improve the whiteness of the stuff together with absorbence likewise improved past times bleaching process. During scouring of cotton fiber all impurities are removed except the natural colouring affair leaving stuff satisfactorily absorbent.
3.4.1 Recipe:
- Wetting agent - 1%
- Hydrogen peroxide - 2-8%
- Soda ash - 0.5 -1%
- Caustic soda - 0.5 %
- Sodium silicate - 1 to 2%
- Temperature - 80-85°c
- PH - 10 to 11.5
- Duration - 1-2 hours
The given sample is weighed past times using electronic residual
↓
Prepare the bleaching bathroom alongside the required amount of wetting agent,hydrogen peroxide,sodium silicate,soda ash,caustic soda using 1:20 stuff to liquor ratio
↓
The bleaching bathroom is good stirred
↓
Adjust the PH of the bathroom 10.5 to 11.0 past times adding sodium carbonate
↓
Then heighten the temperature to 50°c.enter the good scoured cloth into this bath,work for 10 mins
↓
Then heighten the temperature to 85°c together with proceed the bleaching bathroom for 2 hours
↓
Finally take away the cloth launder good together with dried.
↓
Prepare the bleaching bathroom alongside the required amount of wetting agent,hydrogen peroxide,sodium silicate,soda ash,caustic soda using 1:20 stuff to liquor ratio
↓
The bleaching bathroom is good stirred
↓
Adjust the PH of the bathroom 10.5 to 11.0 past times adding sodium carbonate
↓
Then heighten the temperature to 50°c.enter the good scoured cloth into this bath,work for 10 mins
↓
Then heighten the temperature to 85°c together with proceed the bleaching bathroom for 2 hours
↓
Finally take away the cloth launder good together with dried.
3.4.3 After treatment:
After bleaching of cotton fiber alongside hydrogen peroxide is over, a residue Of hydrogen peroxide may left out inward the cloth
↓
Take 1-3% catalase enzyme together with care for the stuff for a menstruation of fifteen mins at room temperature alongside neutral PH.
↓
Then mutual depression temperature launder neutralizing alongside 0.5% acetic acid
↓
Finally thoroughly launder together with dry.
↓
Take 1-3% catalase enzyme together with care for the stuff for a menstruation of fifteen mins at room temperature alongside neutral PH.
↓
Then mutual depression temperature launder neutralizing alongside 0.5% acetic acid
↓
Finally thoroughly launder together with dry.
3.5 CONVENTIONAL METHOD OF DYEING USING SALT
3.5.1 Recipe for dyeing process:
Dyes | Shade % | Wetting agent % | Salt inward gpl | Soda ash inward gpl | Levelling agent | Temp °c | Time |
Red HE3B(120) | 1 % | 1-2 % | 20 gpl | 10 gpl | 1-2 gpl | 80-85°c | 1 – 2 hours |
2 % | 1-2 % | 30 gpl | 20 gpl | 1-2 gpl | |||
3 % | 1-2 % | 40 gpl | 30 gpl | 1-2 gpl | |||
Red HE8B(1520 | 1 % | 1-2 % | 20 gpl | 10 gpl | 1-2 gpl | 80-85°c | 1 – 2 hours |
2 % | 1-2 % | 30 gpl | 20 gpl | 1-2 gpl | |||
3 % | 1-2 % | 40 gpl | 30 gpl | 1-2 gpl | |||
Yellow HE4R(81) | 1 % | 1-2 % | 20 gpl | 10 gpl | 1-2 gpl | 80-85°c | 1 – 2 hours |
2 % | 1-2 % | 30 gpl | 20 gpl | 1-2 gpl | |||
3 % | 1-2 % | 40 gpl | 30 gpl | 1-2 gpl | |||
Navy blueish HEGN(198) | 1 % | 1-2 % | 20 gpl | 10 gpl | 1-2 gpl | 80-85°c | 1 – 2 hours |
2 % | 1-2 % | 30 gpl | 20 gpl | 1-2 gpl | |||
3 % | 1-2 % | 40 gpl | 30 gpl | 1-2 gpl | |||
Blue HERD(160) | 1 % | 1-2 % | 20 gpl | 10 gpl | 1-2 gpl | 80-85°c | 1 – 2 hours |
2 % | 1-2 % | 30 gpl | 20 gpl | 1-2 gpl | |||
3 % | 1-2 % | 40 gpl | 30 gpl | 1-2 gpl |
3.5.2 Calculation:
Material to liquor ratio = 1:20 = cloth weight inward gm × 20 (liquor ratio)
Shade % × weight of the cloth inward gm
Amount of dye solution(ml) = --------------------------------------------------------------------
Stock solution %
Required amount (g/l) ×liquor ratio ×sample weight
Amount of tabular array salt (gm) = -------------------------------------------------------------------------------
K
Required amount(g/l) × liquor ratio × sample wt.
Amount of chemic solution(ml) = -----------------------------------------------------------------------
K × conc.of stock solution %
Water = 5 × 20 = 100 ml
Required quantity of dye solution =(1% × 5 ) / (1%) =5 ml
Required quantity of tabular array salt =(20 gpl ×20 ×5) / (1000) = 2gm
Required quantity of soda ash =(30 gpl ×20 ×5) / (1000) =3 gm
Required quantity of levelling agent = (1gpl ×20×5) / (1000) = 0.1 gm
Required quantity of wetting agent =(1 ×5) / (1) =5 ml
Required quantity of H2O =Total amount of water(M:L:R) -dyes = 100-10 =90 ml
3.5.3 Procedure:
The required quantity of dyestuff is pasted alongside footling quantity of mutual depression temperature H2O
↓
together with so dilution of hot H2O having the temperature of virtually 80°c alongside stirring together with filteration if necessary.
↓
Prepare the dye bathroom alongside the required quantity of dyestuff inward 1:20 M:L:R at 40°c .enter the good scoured/bleached wetted cotton fiber stuff into the hot dye bathroom together with piece of work the stuff for fifteen mins.
↓
Then temperature of the bathroom is gradually raised to 70-80°c.after fifteen mins add together the required quantity of pre-dissolved mutual tabular array salt preferably inward 3 or 4 portions over a menstruation of 30-45 mins.
↓
then add together the required quantity of soda ash into the dye bathroom for fixation of the dyestuff inward the fibre together with piece of work farther abourt xxx mins during the add-on of soda ash the dye bathroom temperature should non live below 80°c.
↓
Then take away the cloth from the dye bathroom together with washed,rinsed well.
↓
together with so dilution of hot H2O having the temperature of virtually 80°c alongside stirring together with filteration if necessary.
↓
Prepare the dye bathroom alongside the required quantity of dyestuff inward 1:20 M:L:R at 40°c .enter the good scoured/bleached wetted cotton fiber stuff into the hot dye bathroom together with piece of work the stuff for fifteen mins.
↓
Then temperature of the bathroom is gradually raised to 70-80°c.after fifteen mins add together the required quantity of pre-dissolved mutual tabular array salt preferably inward 3 or 4 portions over a menstruation of 30-45 mins.
↓
then add together the required quantity of soda ash into the dye bathroom for fixation of the dyestuff inward the fibre together with piece of work farther abourt xxx mins during the add-on of soda ash the dye bathroom temperature should non live below 80°c.
↓
Then take away the cloth from the dye bathroom together with washed,rinsed well.
3.5.4 Soaping:
In companionship to take away the loosely adhered dye particles together with to improve the fastness belongings of dyeing the stuff .
↓
soaping handling alongside 1-5 gpl of lather at boiling status for xxx mins .then launder ,rinse good together with dry out for shade.
↓
soaping handling alongside 1-5 gpl of lather at boiling status for xxx mins .then launder ,rinse good together with dry out for shade.
3.6 TREATMENT OF FABRIC WITH POLYACRYLAMIDE
3.6.1 Preparation of polyacrylamide gel:
Crustacean shells
↓
Size reduction
↓
Protein separation
↓
NAOH washing
↓
Demineralization of HCL
↓
Washing together with watering
↓
Discoloration
↓
Chitin
↓
Deacetylation(NAOH)
↓
Washing together with watering
↓
Polyacrylamide
↓
Size reduction
↓
Protein separation
↓
NAOH washing
↓
Demineralization of HCL
↓
Washing together with watering
↓
Discoloration
↓
Chitin
↓
Deacetylation(NAOH)
↓
Washing together with watering
↓
Polyacrylamide
3.6.2 Properties of polyacrylamide:
- Linear polyamine
- Reactive Amino Groups
- Reactive Hydroxyl Groups are available
- Water soluble together with positively accuse at acidic PH.
- Solution properties of chitosan inward costless Amine (-NH2) shape soluble inward acidic solution.
- Insoluble at pH’s > 6.5 Insoluble inward H2SO4
- Limited solubility inward H3PO4
- Insoluble inward many organic solvents
- Soluble at pH < 6.5
- Form glutinous solutions
- Solution shear thinning, forms gels alongside polyanions
- Will rest soluble inward some alcohol-water mixture.
- Chitosan is a linear polyamine (poly-o-glucosamine) alongside reactive hydroxyl together with amine group. Biocompatility, Cicatrizing,Anti-cholesterolemic agent,Chelation agent,
- Biodegradable, Strengthening the immunity, Antimicrobial activity, Deodrant properties of Chitosan, Water Treatment together with Pollution Control.
The cloth sample was desized using the acid desizing method. The cloth was scoured past times the alkali method using a touchstone procedure. Then, it was subjected to a bleaching physical care for using hydrogen peroxide equally the bleaching agent.
3.6.5 Pretreatment of cotton fiber alongside polyacrylamide
The gray cotton fiber cloth are treated Pre-washed cotton fiber fabrics were immersed for fifteen to 20 minutes inward the polyacrylamide gel alongside different concentrations separately: 5%, 10%, 15%. The padding processes were together with so completed alongside pick upward weight of about 80%. Finally, the cotton fiber fabrics were dried at fourscore °C for 3 min together with cured at 150 °C for 3 min together with finally rinsed alongside warm H2O (40 ºC) for 1 min. Finally cloth rinsed alongside running mutual depression temperature H2O together with dried again.
3.6.6 Recipe:
Sl.no | Polyacrylamide concentration (%) | Wetting agent (%) |
1 | 5 % | 1-2 % |
2 | 10 % | 1-2 % |
3 | 15 % | 1-2 % |
3.6.7 Calculation:
Material to liquor ratio = 1:20 = cloth weight inward gm × 20 (liquor ratio)
= 5 × 20 =100 ml
Amoint of polyacrylamide solution(ml) = (Req. % × wt. of the cloth inward gm)/(stock sol.%)
= ( 5 × 5 ) / (1%) = 25 ml
Amount of wetting agent = (Req.gpl×liquor ratio×wt.of sample)/(1000)
= (1×20×5 )/(1000) =0.1 ml
Required quantity of H2O =Total amount of water(M:L:R) -auxiliars
= 100- 27 =75 ml
3.6.8 Procedure:
The polyacrylamide solution are prepared at different concentration similar 5%,10%,10%
↓
The bleached cotton fiber cloth are immersed inward polyacrylamide solution at neutral status for15-20mins
↓
The cloth are squeezed past times 2 bowls or 3 bowls padding mangle
↓
Then cloth are dried at 80°c for 3 mins
↓
The dried cloth are cured at 130-150°C for 3 mins
↓
The cured cloth rinsed alongside warm H2O (40°c) for 1mins
↓
The cloth are rinsed alongside mutual depression temperature H2O
↓
Finally dried.3.6.9 REACTION OF POLYACRYLAMIDE THROUGH CELLULOSE:
 |
| Figure 3.4: Structure of polyacrylamide |
 |
Then pretreated cloth are dryed at 80°c suitable temperature together with cured at 130°c together with so the cloth are dyed alongside reactive dyes without tabular array salt .this physical care for to eliminate the electrolyte to the dyeing process.
3.7 UNCONVENTIONAL DYEING PROCESS WITHOUT SALT
3.7.1Recipe for dyeing process:
Dyes | Shade % | Wetting agent % | Soda ash inward gpl | Levelling agent | Temp °c | Time |
Red HE3B(120) | 1 % | 1-2 % | 10 gpl | 1-2 gpl | 80-85°c | 1-2 hour |
2 % | 1-2 % | 20 gpl | 1-2 gpl | |||
3 % | 1-2 % | 30 gpl | 1-2 gpl | |||
Red HE8B(152) | 1 % | 1-2 % | 10 gpl | 1-2 gpl | 80-85°c | 1-2 hour |
2 % | 1-2 % | 20 gpl | 1-2 gpl | |||
3 % | 1-2 % | 30 gpl | 1-2 gpl | |||
Yellow HE4R(81) | 1 % | 1-2 % | 10 gpl | 1-2 gpl | 80-85°c | 1-2 hour |
2 % | 1-2 % | 20 gpl | 1-2 gpl | |||
3 % | 1-2 % | 30 gpl | 1-2 gpl | |||
Navy blueish HEGN(198) | 1 % | 1-2 % | 10 gpl | 1-2 gpl | 80-85°c | 1-2 hour |
2 % | 1-2 % | 20 gpl | 1-2 gpl | |||
3 % | 1-2 % | 30 gpl | 1-2 gpl | |||
Blue HERD(160) | 1 % | 1-2 % | 10 gpl | 1-2 gpl | 80-85°c | 1-2 hour |
2 % | 1-2 % | 20 gpl | 1-2 gpl | |||
3 % | 1-2 % | 30 gpl | 1-2 gpl |
3.7.2 Procedure:
The required quantity of dyestuff is pasted alongside footling quantity of mutual depression temperature H2O
↓
Then dilution of hot H2O having the temperature of virtually 80°c alongside stirring together with filteration if necessary.
↓
Prepare the dye bathroom alongside the required quantity of dyestuff inward 1:20 M:L:R at 40°c .enter the good scoured/bleached wetted cotton fiber stuff into the hot dye bathroom together with piece of work the stuff for fifteen mins.
↓
Then temperature of the bathroom is gradually raised to 70-80°c.after 15mins required quantity of 1st one-half portion of soda ash added to the dye bathroom for fixation.
↓
Then add together the 2nd one-half portion of soda ash into the dye bathroom for fixation of the dyestuff inward the fibre together with piece of work farther abourt xxx mins during the add-on of soda ash the dye bathroom temperature should non live below 80°c.
↓
Then take away the cloth from the dye bathroom together with washed,rinsed well.
3.7.3 Soaping:
In companionship to take away the loosely adhered dye particles together with to improve the fastness belongings of dyeing the stuff .
↓
Soaping handling alongside 1-5 gpl of lather at boiling status for xxx mins .then launder ,rinse good together with dry out for shade.
3.8 TESTING METHODS
The Dyed samples are tested such equally washing fastness, rubbing fastness, calorie-free together with perspiration fastness, Delta E value together with biological oxygen demand, chemic oxygen demand together with full dissolved solids equally per touchstone methods. AATCC, ASTM, ISO Test standards are followed for analyzing the fastness properties together with tin give the axe live assessed past times using Grey Scale.
3.8.1 Measurement of washing fastness
Principle of Testing:
Influenza A virus subtype H5N1 sample of textile inward the shape of cloth is inward contact alongside a slice of specified next fabrics inward mechanically agitated inward a lather or soap-soda solution, rinsed together with dried. The alter inward color of the specimen together with the staining of the next fabrics are assessed alongside a gray scales.
Sampling:
The sample should live a representative sample or ane agreed to betwixt the buyer together with the seller.
Apparatus:
Influenza A virus subtype H5N1 suitable mechanical washing machine alongside the next requirements to live used.
- Water bathroom containing a rotor past times agency of which contains 500ml capacity is rotated at a speed of 40+ revolutions/ min. The containers may live stainless steel or glass.
- Means of thermostatically controlling the temperature of the H2O bathroom so equally to maintain the temperature an accuracy of twoscore + 2oC.
The washing fastness of the dyed textiles may live performed nether 5 different touchstone essay weather condition equally shown inward the tabular array below.
| Washing Test No. | Second Adjacent cloth | Soap solution conc. | Soda ash conc. | Tempof essay o C | Time of handling | Steel balls of 6mm dia | M : L ratio | IS Test Number |
| 1. | Wool 10x4cm | 5 g/l | NIL | 40 + 2oC | 30 min. | --- | 1 : 50 | IS687:1979 |
| 2. | Wool 10x4cm | 5g/l | NIL | 50 + 2oC | 45 min. | --- | 1 : 50 | IS3361:1979 |
| 3. | Wool 10x4cm | 5 g/l | 2 g/l | 60 + 2oC | 30 min. | --- | 1 : 50 | IS764:1979 |
| 4. | Viscose 10x4cm | 5 g/l | 2 g/l | 95 + 2oC | 30 min. | 10 | 1 : 50 | IS765:1979 |
| 5. | Viscose 10x4cm | 5 g/l | 2 g/l | 95 + 2oC | 4 hrs. | 10 | 1 : 50 | IS3417:1979 |
As per the specifications the essay specimens of size 10 x 4 cm is taken. Then 2 pieces of next fabrics of 10 x 4 cm( equally per the given specifications together with fabrics type) are taken. Then a composite specimen is prepared past times keeping the essay specimen inward betwixt the 2 pieces of un dyed cloth together with stitched about at the 4 edges equally shown inward figure. Necessary lather soda solution is prepared equally per the M :L ratio 1 :50. The specimen is kept inward the container of the washing machine alongside the lather or lather soda solution. The containers are closed together with washing is carried for xxx minutes alongside twoscore o C. Remove the composite specimen , rinse it twice inward mutual depression temperature H2O together with mutual depression temperature running H2O for 10 minutes.
Remove the stitches along the 2 long sides together with ane small side. Open out the composite specimen together with dried it inward hot air oven at threescore o C or at room temperature.
Evaluation of color fastness to washing:
The composite specimens containing the specimen is evaluated alongside the gray scale for a. Change inward colour, b. Staining
The gray scale consists of nine pairs of touchstone gray chips, each twosome representing the deviation inward colours (shade together with strength) corresponding to the numerical fastness rating. This ratings may live described inward qualitative terms equally follows.
| Rating | Qualitative Description |
| 5 | Excellent |
| 4 – 5 | Very Good to Excellent |
| 4 | Very Good |
| 3 – 4 | Good to real good |
| 3 | Good |
| 2 – 3 | Fair to Good |
| 2 | Fair |
| 1 – 2 | Poor to fair |
| 1 | Poor |
Similarly some other laid of gray scale for staining
3.8.2 MEASUREMENT OF RUBBING FASTNESS
Principle: Specimens of textiles are rubbed alongside dry out rubbing cloth together with alongside wet rubbing cloth past times agency of rubbing finger of specified dimensions. The staining of rubbing cloth is assessed alongside the gray scale for staining. The ratings are assessed for dry out rubbing together with wet rubbing fastness of the specimen.procedure followed past times ISO method. [ISO-105-X12]
Sampling: The sample shall live selected so equally to live the representative of sample of the lot or equally agreed to betwixt the buyer together with seller.
Apparatus:
 |
| Figure3.8: Crock meter |
Rubbing cotton fiber cloth: This should live bleached without whatever goal together with cutting it into a size of 5 x 5 cm.
Test Specimen: It should non live less than fourteen cm x 5 cm. Two pieces are to live used ane inward warp way together with ane inward weft way for dry out rubbing test. Similarly for wet rubbing essay 2 pieces are to live used.
Procedure: Fix the essay specimen to the rubbing device past times agency of clamps such that the long administration of the specimen follows the rail of the device.
a). Dry rubbing: Dry rubbing cloth is fixed apartment inward house of over the halt of the finger of the device. Fix the essay specimen to the rubbing device past times agency of clamps such that the long administration of the specimen follows the rail of the device. Operate the apparatus together with rub the essay specimen to together with fro inward a straight line along the rail to 10 times alongside the downward strength of nine Newton’s. Remove the essay specimen out together with compare it alongside the touchstone cloth for staining test. Assign the rating past times using the gray scale for staining. Each essay is conducted for warp together with weft way directions of the fabric.
b). Wet rubbing: Fix the essay specimen to the rubbing device past times agency of clamps such that the long administration of the specimen follows the rail of the device. Influenza A virus subtype H5N1 touchstone cloth is wetted for 10 min. inward a disc containing distilled H2O to take-up virtually 100%. Then it is fixed apartment over the halt of the finger of the testing device. Operate the apparatus together with rub the essay specimen to together with fro inward a straight line along the rail to 10 times alongside the downward strength of nine Newton’s. Remove the essay specimen out together with compare it alongside the touchstone cloth for staining test. Assign the rating past times using the gray scale for staining. Each essay is conducted for warp together with weft way directions of the fabric.
Evaluation of color fastness to rubbing:
The composite specimens containing the specimen is evaluated alongside the gray scale for a. Change inward color b. Staining
The gray scale consists of nine pairs of touchstone gray chips, each twosome representing the deviation inward colours (shade together with strength) corresponding to the numerical fastness rating. This ratings may live described inward qualitative terms equally follows.
| Rating | Qualitative Description |
| 5 | Excellent |
| 4 – 5 | Very Good to Excellent |
| 4 | Very Good |
| 3 – 4 | Good to real goodness |
| 3 | Good |
| 2 – 3 | Fair to Good |
| 2 | Fair |
| 1 – 2 | Poor to fair |
| 1 | Poor |
3.8.3 MEASUREMENT OF FASTNESS TO ALKALI, ACID PERSPIRATION:
Principle: Specimens of the Textiles inward contact alongside next fabrics are treated alongside 2 different solutions of acid together with alkali immerse it for xxx minutes together with the removed .The materials is uniformly squeezed together with placed nether a specified charge for 4 hrs inward betwixt the 2 acrylic sheets of perspirometer for 4 hours .Then the samples are removed from the plates, stitches are removed on 3 sides of the samples, together with so dried together with compared for the shade variation inward the gray scales for assessment of shade variations.
Reagents: As human perspiration may live acid or alkali 2 solutions are prepared.
a. Alkali solution : Freshly prepared ,containing the next chemicals per litre
- 0.5 g of Sodium chloride (Nacl) together with
- 5g of disodium hydrogen orthophosphate dodecahydrate
5g 1- hisitidine monohydrochloride monohydrate
5g of sodium chloride together with 5g of sodium dihydrogen orthophosphate dehydrate
This solution is brought to pH 5.5 alongside acetic acid solution.
Preparation of essay Specimens:
If the textile to live tested inward the shape of fabric, house a specimen 10 x 4 cm inward size betwixt 2 10x 4 cm pieces of the 2 kinds of next fabrics together with sew together along ane of the shorter sides to shape a composite specimen. Two such composite specimens are required.
Procedure : Wet ane of the composite specimen thoroughly inward the element of group I solution using a liquor to specimen ratio of 50 : 1 together with allow it to rest inward the solution at room temperature for xxx minutes. press together with displace it from fourth dimension to fourth dimension to ensure goodness together with uniform penetration of the liquor .Pour off the liquor together with wipe the excess liquor offthe specimen betwixt 2 drinking glass rods.
Then palce the composite specimen betwixt 2 drinking glass or acrylic resin paltes nether a strength of 5.1 kgs (as the specimen is 10 x 4 cm inward size ) inward a perspirometer.Keep the perspirometer inward the air oven at 37 + /- 2 ○C for hours.
Open out the composite specimen past times breaking the stitches on all the sides except ane of the shorter sides. Dry the 3 parts inward air at temperature non to a greater extent than than 60°C
With the 3 parts inward contact only at the remaining line of stitching.
Treat the 2nd composite inward acid solution equally noted inward a higher house instead of element of group I solution. Evaluate the alter inward color of the treated essay specimens together with the grade ofstaining of the 2 pieces of next cloth alongside assist of the gray scale together with assign theratings, taking aid to start cool the essay specimens together with the next fabrics to room temperature. In instance of uncertainty inward the rating given past times an observer, the accessestment should live done past times at to the lowest degree 3 observers together with the overall average rating should live reported.
 |
| Figure 3.10: Perspirometer |
The essay specimen is evaluated for staining together with and so ratings are accessed.
3.8.4 MEASUREMENT OF LIGHT FASTNESS
Dyed or Printed stuff is exposed inward 24-hour interval calorie-free / artificial xenon arc light calorie-free to assess the Light fastenss Property.It is assessed on a scale of 8 (1……..8): 1 representing the to the lowest degree fastness together with 8 the best.
Principle:
A specimen of the textile is exposed to day-light nether prescribed conditions, including protection from rain, along alongside 8 dyed wool standards. The fastness is assessed past times comparison the alter inward color of the specimen alongside that of the standards.
Blue wool standards produced inward Europe are identified past times the numerical designation 1 to 8. These standards are blueish wool cloths dyed alongside the dyes listed below. The make from 1 (very depression calorie-free fastness) to 8 (very high calorie-free fastness)
List of touchstone wool dyes:
| S.No | LF dye rating | CI Designation | Chemical class |
| 1 | Acilan Brilliant Blue FFR | CI Acid Blue 104 | Triaryl methane |
| 2 | Acilan Brilliant Blue FFB | CI Acid Blue 109 | Triaryl methane |
| 3 | Coomassie Brilliant Blue R | CI Acid Blue 83 | Triaryl methane |
| 4 | Supramine Blue EG | CI Acid Blue 121 | Azine |
| 5 | Solway Blue EG | CI Acid Blue 47 | Anthraquinonoid |
| 6 | Alizarin Light Blue 4GL | CI Acid Blue 23 | Anthraquinonoid |
| 7 | Soledon Blue 4 BC pdr | CI Solublised Vat Blue 5 | Indigoid |
| 8 | Indigosol Blue AGG | CI Solublised Vat Blue 8 | Indigoid |
The dyeing strength inward both of the touchstone systems of 1 to 8 are omitted because of sure enough manufacturing problems but the sets of the touchstone dyeing are marketed after getting them matched inward depth of shade together with fading charge per unit of measurement alongside those of the original set.
Assessment of calorie-free fastness:
Compare the alter inward color of the essay specimen alongside the changes which take hold occurred inward the touchstone patterns. The calorie-free fastness of the specimen is the number of the touchstone pattern which shows similar changes inward color (Visual contrast betwixt the exposed together with unexposed portions of the specimen). If the specimen shows changes inward color, about one-half way betwixt 2 standards, one-half rating may live given for eg, 3 – 4.
 |
| Figure 3.11: Grey scale |
 |
| Table 3.12. Fastness raing |
Colour tin give the axe live defined equally the number on the encephalon of an observer when an object is viewed inward the presence of a calorie-free source.
In textile industries, the desired color is obtained past times mixing 3 or 4 dyes. The most of import work inward the manufacture is how to build it at the perfect fit to the customer’s samples using minimum amount of chemicals together with dyes. This chore is usually attended past times an goodness dyeing original who decides the 3 or 4 dyes color recipe to reproduce a given shade. The dyeing original maintains the tape of his sense inward “shade Bank” together with selects ane of the color recipes, which may live unopen to the standard. He, then, makes necessary changes past times trial together with mistake method to obtain the exact match.most appropriate recipe depending on the terms of production together with lineament of the products required. This technique is known equally Computer Aided Color Matching.
A reckoner color matching arrangement comprises of,
- A spectrophotometer
- A personnel reckoner
- A color matching software
Light source:
The calorie-free source illuminates the specimen. The calorie-free source used inward spectrophotometers are tungsten halogen, xenon flash, calorie-free emitting diode, emits the calorie-free inward the visible region.
Integrating sphere: It is coated alongside barium sulphate, to copy the weather condition of daylight
Related:
Detector:
A sensor which detects liberate energy of the calorie-free source that is reflected dorsum past times object
Signal processor or microprocessor:
In optical sensor the electrical signal is generated proportional to calorie-free liberate energy reflected from the sample. These electrical signals corresponding to different wavelengths are fed to microprocessor. The microprocessor converts the analogue signal from optical sensor into digital shape to compute together with display several color parameters next CIE recommendations. (CIE- committee International del’Eclairage or International Commission of Illumination).For textile Samples the measurements inward a spectral make from 400 to 700nm at 20nm interval is used.
The color matching software consist of the next modules / programmes:
Quality assurance:
All QA applications involve comparison batches against a standard.
QA functions inward the next applications:
- colour deviation assessment for approved touchstone together with a batch
- whiteness / yellowness index for assessing whiteness of OBA treated samples together with yellowness for white textile substrates.
- pass / neglect status for batch production
Generate K/S information using representative substrates together with processes. Analyses K/S information outputs for various colorant properties
Formulation:
Match novel equally good equally electrical flow shades together with obtain formulation for terms saving. It performs color matching for a given target using selected dyes.
Batch correction: Use batch correction for laboratory together with production correction. It volition assist inward reducing trail together with mistake method.
Colour maker:
Use color maker computer program for generating novel shades.
Shade Library:
Stores all lab together with productions shades into computer.
File management:
Use file administration for creating a user data. maintain dorsum upward of information files using this program.
Instrument :
 |
| Figure 3.13.Computer color matching musical instrument |
3.8.6 MEASUREMENT OF BIOLOGICAL OXYGEN DEMAND (BOD)
BOD:
Biological oxygen demand is the amount of dissolved oxygen needed(i.e. demanded) past times aerobic biological organisms to suspension downward organic stuff nowadays inward a given H2O sample at a sure enough temperature over a specific fourth dimension period.
Apparatus:
- Incubator
- Burette
- Pipette
- Conical flask
- BOD bottles
- Manganese,sulphate
- Sodium azide
- Potassium iodide
- Sodium hydroxide
- Starch,potassium fluoride
- Sodium thiosulphate
BOD is the amount of oxygen utilized past times microorganisms inward biological physical care for that suspension downward organic affair inward water.it is a mensurate of organic pollutant load.greater the oxidizable organic affair inward water,the greater volition live the BOD,thus ,the strength of waste product is expressed inward terms of BOD. BOD, is of import to know the amount of organic affair nowadays inward the waste product together with that the quantity of oxygen required for its stabilization .the BOD values are thus real useful inward physical care for designed to mensurate handling flora efficiency together with operation.
The root of organic compounds are consummate the human excreta,vegetables,animal waste, together with industrial waste.the amount of organic affair nowadays inward H2O shows the amount of biological oxygen demand. large amount of sewage introduced into H2O trunk do non larn diluted sufficiently. Microorgnisms utilization upward all the oxygen inward the H2O to oxidize organic affair equally a resultant of which H2O becames useless for drinking together with for the other purposes.
The consummate degradation of organic affair may take hold 20 to xxx days .simple organic compounds similar glucose are almost completely oxidized inward 5 days spell domestic sewage is oxidized to 65 %in this period. Complex organic compounds larn oxidized only upto 40% inward the same period. The 20 to xxx days menstruation is of to the lowest degree significance inward practice.therefore ,the BOD essay has been developed for 5 days at 20°c .BOD inward full general gives a qualitative index of organic marrow which are degraded chop-chop inward a small menstruation time.
The BOD value is mensurate past times measuring the deviation of dissolved oxygen (DO) of the same H2O sample inward 5 days.DO nowadays inward H2O is calculated past times using the formula.
Dissolved oxygen (mg/l) = ( A.N.8 ) /(S-C) ×1000
Where,
A = ml of 0.025 north thiosulphate solution consumed
north = normality of touchstone thiosulphate solution
C = Total book of potassium manganese, sulphate, alkali iodide azide, Potassium fluoride
due south = book of H2O sample
Procedure:
The H2O samples was collected inward 2 bottles .one BOD bottle was incubated inward BOD incubator for 5 days or 3 days at 20*c spell DO of some other BOD was determined on the 1 st day. After 5 days or 3 days ,another BOD bottles was removed from the incubator together with DO was determined.difference inward DO was calculated ,which gave the mensurate of BOD.
Calculation:
BOD of H2O is calculated past times using the formula,
DO 1 – DO 5
BOD (mg/l) = ------------------------
P
Where,
DO 1-DO inward H2O sample inward 1 st day.
DO 5- DO inward H2O sample inward 5 days.
P = decimal volumetric fraction
A.N.8
Dissolved oxygen (mg/l) = ------------------ × K
due south – C
Where,
A = ml of 0.025 north thiosulphate sil.consumed
north = normality of touchstone thiosulphate solution
C = Total book of potassium manganese, sulphate alkali iodide azide, Potassium fluoride
due south = book of H2O sample
3.8.7 MEASUREMENT OF CHEMICAL OXYGEN DEMAND (COD)
Introduction:
Chemical Oxygen Demand (COD) essay determines the oxygen requirement equivalent of organic affair that is susceptible to oxidation alongside the assist of a strong chemic oxidant. It is an important, rapidly measured parameters equally a agency of measuring organic strength for streams together with polluted H2O bodies. The essay tin give the axe live related empirically to BOD, organic carbon or organic affair inward samples from a specific source taking into occupation organization human relationship its limitations. The essay is useful inward studying performance evaluation of wastewater handling plants together with monitoring relatively polluted H2O bodies. COD decision has wages over BOD determination. COD results tin give the axe live obtained inward 3-4 hrs equally compared to 3-5days required for BOD test.
Open Reflux method
Principle
The opened upward reflux method is suitable for a broad make of wastes alongside a large sample size. The dichromate reflux method is preferred over procedures using other oxidants (e.g. potassium permanganate) because of its superior oxidizing ability, applicability to a broad diversity of samples together with ease of manipulation. Oxidation of most organic compounds is upward to 95-100% of the theoretical value.
The organic affair gets oxidised completely past times potassium dichromate (K2Cr2O7) alongside silvery sulphate equally catalyst inward the presence of concentrated H2SO4 to create CO2 together with H2O. The excess K2Cr2O7 remaining after the reaction is titrated alongside ferrous ammonium sulphate [Fe (NH4)2(SO4)2]. The dichromate consumed gives the oxygen (O2) required for oxidation of the organic matter. The chemic reactions involved inward the method are equally under:
a. 2K2Cr2O7 + 8 H2SO4 ® 2 K2 SO4 + 2Cr2(SO4)3 + 8 H2O + 3O
b. C6H12O6 + 6O2 ® 6CO2 + 6H2O
c. Cr2O7-- + 6Fe++ + 14H+ ® 6Fe+++ + 2Cr3+ + 7H2O
Apparatus together with equipment
- 250 or 500mL Erlenmeyer flask alongside touchstone (24/40) tapered drinking glass joints
- Friedrich’s reflux condenser (12 inch) alongside touchstone (24/40) tapered drinking glass joints
- Electric hot plate or six-unit heating shelf
- Volumetric pipettes (10, 25, together with 50mL capacity)
- Burette, 50mL alongside 0.1mL accuracy
- Burette stand upward together with clamp
- Analytical balance, accuracy 0.001g
- Spatula
- Volumetric flasks (1000mL capacity)
- Boiling beads, drinking glass
- Magnetic stirrer together with stirring bars.
a. Standard potassium dichromate solution, 0.25N (0.04167 M): Dissolve 12.259g K2Cr2O7 dried at 103°C for 24h inward distilled H2O together with dilute to 1000mL. Add virtually 120mg sulphamic acid to take hold aid of 6 mg/L NO2 – N.
b. Sulphuric acid reagent: Add 10g of Ag2SO4 to 1000mL concentrated H2SO4 together with permit stand upward for ane to 2 days for consummate dissolution.
c. Standard ferrous ammonium sulphate approx. 0.25N (0.25M): Dissolve 98g Fe(NH4)2(SO4)2.6H2O inward virtually 400mL distilled water. Add 20mL concentrated H2SO4 together with dilute to 1000mL.
d. Ferroin indicator: Dissolve 1.485g 1, 10-phenanthroline monohydrate together with 695mg FeSO4.7H2O inward distilled H2O together with dilute to 100mL.
e. Mercuric Sulphates: HgSO4, crystals, analytical grade
f. Potassium hydrogen phthalate (KHP) Standard: Dissolve 425mg lightly crushed dried potassium hydrogen phthalate (HOOC.C6H4.COOK) inward distilled H2O together with dilute to 1000mL. This solution has a theoretical COD of 500μg O2/mL. This solution is stable when refrigerated, upward to 3 months inward the absence of visible biological growth.
Sample collection, preservation together with storage
Preferably collect samples inward drinking glass bottles. Homogenise samples containing settleable solids. If in that location is delay inward collection together with analysis, save sample past times acidification to pH≤2 using concentrated H2SO4. Samples tin give the axe live preserved for maximum vii d.
Procedure
Sample preparation: All samples high inward solids should live blended for 2 minutes at high speed together with stirred when an aliquot is taken for analysis. Select the appropriate book of sample based on expected COD range, e.g. for COD make of 50-500 mg/L take hold 25-50mL of sample. Sample book less than 25mL should non live pipetted directly, but serially diluted together with and so a portion of the diluted sample taken. Dilution factor should live incorporated inward calculations.
- 500mL of sample diluted to 1000mL = 0.5mL sample/mL of diluent, 50mL = 25mL of sample.
- 100mL of sample diluted to 1,000mL = 0.1mL sample/mL diluent, 50mL of diluent = 5mL of sample.
- Place 0.4g HgSO4 inward a 250mL reflux sample
- Add 20mL sample or an aliquot of sample diluted to 20mL alongside distilled water. Mix well.
- Add build clean pumic stones or drinking glass beads.
- Add 10mL 0.25N (0.04167M) K2Cr2O7 solution together with mix.
- Add piece of cake 30mL concentrated H2SO4 containing Ag2SO4 mixing thoroughly. This ho-hum add-on along alongside swirling prevents obese acids to escape due to generation of high temperature. Alternatively attach flask to condenser alongside H2O flowing together with and so add together H2SO4 piece of cake through condenser to avoid escape of volatile organic marrow due to generation of heat.
- Mix well. If the color turns green, either take hold fresh sample alongside lesser aliquot or add together to a greater extent than potassium dichromate together with acid.
- Connect the flask to condenser. Mix the contents earlier heating. Improper mixing volition resultant inward bumping together with blow out of flask content.
- Reflux for a minimum of 2 hours. Cool together with and so launder downward condenser alongside distilled water.
- Disconnect reflux condenser together with dilute the mixture to virtually twice its book alongside distilled water. Cool to room temperature together with titrate excess K2Cr2O7 with0.1M FAS using 2-3 drops of ferroin indicator. The abrupt color alter from blueish light-green to reddish chocolate-brown indicates end-point or completion of the titration. After a modest fourth dimension gap, the blue-green color may reappear. Use the same quantity of ferroin indicator for all titrations.
- Reflux blank inward the same way using distilled H2O instead of sample.
Calculations
COD equally mg/L = (a –b) x north x 8000 / mL sample
Where,
a = mL FAS used for blank
b = mL FAS used for sample
N = normality of FAS
8000 = Milieq. wt. of O2 x K
3.8.8 MEASUREMENT OF TOTAL AND DISSOLVED SOLID FROM EFFLUENT WATER SAMPLE
Total dissolve solid
Total Dissolved Solids (often abbreviated TDS) is a mensurate of the combined content of all inorganic together with organic substances contained inward a liquid similar molecular, ionized or micro-granular (colloidal sol) suspended form. The full dissolved solids are modest plenty to filter through a sieve the size of 2 micrometer.
Total dissolved solids parameters are applied for freshwater systems, equally salinity comprises some of the ions constituting likewise define equally TDS. The principal application of TDS is inward the study of H2O lineament for streams, rivers together with lakes, although TDS is non by together with large considered a primary pollutant parameter because it is non straight associated alongside wellness effects. Generally it is an indication of aesthetic (visual) characteristics of drinking H2O together with equally an aggregate indicator of the presence of a broad grouping of chemic contaminants.
Sources of TDS
Primary sources for TDS inward receiving waters are Dyeing outlet H2O together with H2O runoff, leaching of soil contaminant together with effluent discharge from industrial or sewage handling plants. The most mutual chemic constituents are calcium, phosphates, nitrates, sodium, potassium together with chloride, which are found inward food runoff together with H2O runoff.
Measurement of TDS
The 2 principal methods of measuring full dissolved solids are gravimetric together with conductivity. Gravimetric methods are the most accurate together with involve evaporating the liquid solvent to travel out a residue that tin give the axe afterwards live weighed alongside a precision analytical residual (normally capable of 0.0001 gram accuracy). This method is by together with large the best, although it is time-consuming together with leads to inaccuracies if a high proportion of the TDS consists of depression boiling dot organic chemicals, which volition evaporate along alongside the water. If inorganic salts comprise the keen bulk of TDS, gravimetric methods are appropriate.
Electrical electrical conductivity of H2O is straight related to the concentration of dissolved ionized solids inward the water. Ions from the dissolved solids inward H2O create the mightiness for that H2O to acquit an electrical current, which tin give the axe live measured using a conventional electrical conductivity meter or TDS meter. When correlated alongside laboratory TDS measurements, electrical conductivity provides an gauge value for the TDS concentration, usually to within ten-percent accuracy. (A) Total Solids
These are the mensurate of the amount of all kinds of solids. i.e. Suspended, dissolved, volatile etc. inward effluent H2O sample. Total solids tin give the axe live measured equally the residue left after evaporation of the unfiltered sample.
Procedure
b = mL FAS used for sample
N = normality of FAS
8000 = Milieq. wt. of O2 x K
3.8.8 MEASUREMENT OF TOTAL AND DISSOLVED SOLID FROM EFFLUENT WATER SAMPLE
Total dissolve solid
Total Dissolved Solids (often abbreviated TDS) is a mensurate of the combined content of all inorganic together with organic substances contained inward a liquid similar molecular, ionized or micro-granular (colloidal sol) suspended form. The full dissolved solids are modest plenty to filter through a sieve the size of 2 micrometer.
Total dissolved solids parameters are applied for freshwater systems, equally salinity comprises some of the ions constituting likewise define equally TDS. The principal application of TDS is inward the study of H2O lineament for streams, rivers together with lakes, although TDS is non by together with large considered a primary pollutant parameter because it is non straight associated alongside wellness effects. Generally it is an indication of aesthetic (visual) characteristics of drinking H2O together with equally an aggregate indicator of the presence of a broad grouping of chemic contaminants.
Sources of TDS
Primary sources for TDS inward receiving waters are Dyeing outlet H2O together with H2O runoff, leaching of soil contaminant together with effluent discharge from industrial or sewage handling plants. The most mutual chemic constituents are calcium, phosphates, nitrates, sodium, potassium together with chloride, which are found inward food runoff together with H2O runoff.
Measurement of TDS
The 2 principal methods of measuring full dissolved solids are gravimetric together with conductivity. Gravimetric methods are the most accurate together with involve evaporating the liquid solvent to travel out a residue that tin give the axe afterwards live weighed alongside a precision analytical residual (normally capable of 0.0001 gram accuracy). This method is by together with large the best, although it is time-consuming together with leads to inaccuracies if a high proportion of the TDS consists of depression boiling dot organic chemicals, which volition evaporate along alongside the water. If inorganic salts comprise the keen bulk of TDS, gravimetric methods are appropriate.
Electrical electrical conductivity of H2O is straight related to the concentration of dissolved ionized solids inward the water. Ions from the dissolved solids inward H2O create the mightiness for that H2O to acquit an electrical current, which tin give the axe live measured using a conventional electrical conductivity meter or TDS meter. When correlated alongside laboratory TDS measurements, electrical conductivity provides an gauge value for the TDS concentration, usually to within ten-percent accuracy. (A) Total Solids
These are the mensurate of the amount of all kinds of solids. i.e. Suspended, dissolved, volatile etc. inward effluent H2O sample. Total solids tin give the axe live measured equally the residue left after evaporation of the unfiltered sample.
Procedure
- Take an evaporating dish together with dry out it till constant weight together with authorities annotation downward the weight of evaporating dish.
- Take 50 ml of unfiltered good shaken H2O sample inward evaporating dish.
- Put it on H2O bathroom for evaporation or oestrus at 105'c for 1 hr inward an oven.
- After all H2O is evaporated shape the evaporating dish, lay evaporating dish inward decicator for cooling for ane hour.
- Take concluding weight of evaporating dish together with calculate full solids past times given formula
- Initial weight of the evaporating dish (g) = Influenza A virus subtype H5N1
- Final weight of the evaporating dish (g) = B
- Volume of the sample evaporating (ml) = V
CHAPTER 4
RESULTS AND DISCUSSIONS
Cotton cloth are pretreated alongside polyacrylamide solution together with dyed alongside hot build reactive dyes using tabular array salt together with without salt. the essay results are discussed hither alongside different testing results similar washing, rubbing, light, perspiration fastness together with full dissolved solids(TDS),biological oxygen demand(BOD),chemical oxygen demand(COD).
4.1 WASHING FASTNESS
The washing fastness of the samples was tested past times using launder meter.
| Sl.no | Samples | Dyes % | Rating (conventional) | Rating (unconventional) | Description |
| 1 | RED HE3B | 1% | 3-4 | 4 | Moderate to good |
| 2% | 4 | 4-5 | good | ||
| 3% | 4 | 4-5 | Good | ||
| 2 | RED HE5B | 1% | 4 | 3-4 | Moderate to good |
| 1% | 3-4 | 4 | Moderate to good | ||
| 2% | 3-4 | 5 | Good | ||
| 3% | 4 | 4-5 | Good | ||
| 3 | YELLOW HE4R | 1% | 4 | 3-4 | Moderate to good |
| 2% | 4-5 | 4 | Good | ||
| 3% | 4-5 | 5 | Very good | ||
| 4 | NAVY BLUE HEGN | 1% | 3-4 | 4 | moderate |
| 2% | 4-5 | 4-5 | Moderate to good | ||
| 3% | 4 | 5 | Good | ||
| 5 | BLUE HEGN | 1% | 3-4 | 4 | Moderate to good |
| 2% | 4 | 4-5 | Good | ||
| 3% | 4-5 | 4-5 | Good |
 |
| Figure 4.2 .Washing fastness rating value of dyed sample |
The rubbing fastness of the samples was tested past times using crock meter.
| Sl.no | Samples | Dyes % | Rating (conventional) | Rating (unconventional) | Description | ||
| Dry | Wet | Dry | Wet | ||||
| 1 | RED HE3B | 1% | 4 | 2-3 | 4 | 3 | Moderate to good |
| 2% | 4 | 3 | 4-5 | 3 | Good | ||
| 3% | 4-5 | 3-4 | 4-5 | 3-4 | Good | ||
| 2 | RED HE5B | 1% | 4 | 3 | 4 | 3 | good |
| 2% | 4-5 | 3-4 | 5 | 3-4 | Moderate to good | ||
| 3% | 4 | 3-4 | 4-5 | 3-4 | good | ||
| 3 | YELLOW HE4R | 1% | 4-5 | 3 | 5 | 3 | Moderate to good |
| 2% | 5 | 3 | 4-5 | 3 | Good | ||
| 3% | 5 | 3-4 | 5 | 3-4 | Good | ||
| 4 | NAVY BLUE HEGN | 1% | 4 | 3 | 4 | 3 | moderate |
| 2% | 4-5 | 2-3 | 4-5 | 2-3 | good | ||
| 3% | 5 | 3 | 4-5 | 3 | good | ||
| 5 | BLUE HEGN | 1% | 4 | 3 | 4 | 3 | Good |
| 2% | 4-5 | 3-4 | 4-5 | 3-4 | Moderate to good | ||
| 3% | 4-5 | 3 | 4-5 | 3-4 | Good | ||
 |
| Figure 4.4.Rubbing fastness rating value deviation of dyed sample |
The calorie-free fastness of the samples was tested past times 24-hour interval light.
| Sl.no | Samples | Dyes % | Rating (conventional) | Rating (unconventional) | Description |
| 1 | RED HE3B | 1% | 6 | 5-6 | good |
| 2% | 6 | 6 | Very good | ||
| 3% | 5-6 | 6-7 | good | ||
| 2 | RED HE5B | 1% | 6 | 6 | Very good |
| 2% | 6 | 6-7 | good | ||
| 3% | 6 | 6 | good | ||
| 3 | YELLOW HE4R | 1% | 5-6 | 5-6 | good |
| 2% | 6 | 6-7 | good | ||
| 3% | 6 | 6-7 | Very good | ||
| 4 | NAVY BLUE HEGN | 1% | 6 | 6 | good |
| 2% | 5-6 | 6 | good | ||
| 3% | 6 | 6-7 | Very good | ||
| 5 | BLUE HEGN | 1% | 6 | 6 | good |
| 2% | 6 | 6-7 | Very good | ||
| 3% | 6 | 6 | good |
 |
| Figure 4.6 Light fastness rating deviation of dyed sample. |
The perspiration fastness of the samples was tested past times using perspirometer.
| Sl.no | Samples | Dyes % | Rating (conventional) | Rating (unconventional) | Description | ||
| Acid | Alkali | Acid | Alkali | ||||
| 1 | RED HE3B | 1% | 4 | 3-4 | 4 | 3-4 | Moderate to good |
| 2% | 3-4 | 4 | 4-5 | 4-5 | Very good | ||
| 3% | 4 | 4 | 4 | 4 | good | ||
| 2 | RED HE5B | 1% | 4 | 4-5 | 4 | 4-5 | Very good |
| 2% | 4 | 4 | 4 | 4 | Good | ||
| 3% | 5 | 4 | 4-5 | 4 | Good | ||
| 3 | YELLOW HE4R | 1% | 4 | 3-4 | 4 | 3-4 | Good |
| 2% | 3-4 | 4-5 | 3-4 | 4-5 | Moderate to good | ||
| 3% | 4 | 4 | 4 | 4 | Very good | ||
| 4 | NAVY BLUE HEGN | 1% | 5 | 3-4 | 5 | 3-4 | Good |
| 2% | 4 | 4 | 4 | 4-5 | Good | ||
| 3% | 3-4 | 4 | 4 | 4 | Very good | ||
| 5 | BLUE HEGN | 1% | 5 | 4-5 | 5 | 4-5 | Good |
| 2% | 4 | 4 | 4 | 4 | Very good | ||
| 3% | 4 | 3-4 | 4 | 3-4 | Good | ||
 |
| Figure 4.8: Perspiration fastness rating deviation of dyed sample. |
The color strength of the samples was tested past times using spectrophotometer.the delta E is a metric for agreement how the human oculus perceives color difference.Delta E is a unmarried number representing the “distance” betwixt 2 colours.this value measured from reference sample(conventional dyed sample using salt) together with target (unconventional dyed sample without salt) sample.
| Sl.no | Samples | Shade % | Between Delta E (conventional &unconventional dyed sample) |
| 1 | Red HE3B | 1% | 0.48 |
| 2% | 0.70 | ||
| 3% | 0.59 | ||
| 2 | Red HE5B | 1% | 0.67 |
| 2% | 0.58 | ||
| 3% | 0.62 | ||
| 3 | Yellow HE4R | 1% | 0.53 |
| 2% | 0.72 | ||
| 3% | 0.56 | ||
| 4 | Navy blueish HEGN | 1% | 0.58 |
| 2% | 0.49 | ||
| 3% | 0.68 | ||
| 5 | Blue HEGN | 1% | 0.54 |
| 2% | 0.57 | ||
| 3% | 0.60 |
 |
| Figure 4.10: Delta E value deviation of cloth |
Biological oxygen demand is the amount of dissolved oxygen needed (i.e demanded) past times aerobic biological organisms to suspension downward organic stuff nowadays inward a given H2O sample at sure enough temperature over a specific fourth dimension period.The BOD of the samples was tested past times using Titrimetric metod.the BOD bird of conventional dyeing together with unconventional dyeing are shown inward below.
BOD value of dyedoutlet H2O sample:
| Sl..No | BOD 3 days | Conventional Dyeing Outlet - Water Sample | Un Conventional Dyeing Outlet - Water Sample |
| 1 | Biological Oxygen Demand, mg/l 27 deg C, 3 days | 710 mg/l | 440 mg/l |
 |
| Figure 4.12: BOD value deviation of dyed outlet H2O sample |
Chemical oxygen demand(COD) is a measuring of the oxygen required to oxidize soluble together with particulate organic affair inward water.this value dot the amount of oxygen needed to oxidize all organic marrow inward water.
The COD of the samples was tested past times using Open Reflux method .the COD bird of conventional dyeing together with unconventional dyeing are shown inward below.
COD value of dyed outlet H2O sample:
| S.NO | COD | Conventional Dyeing Outlet - Water Sample | Unconventional Dyeing Outlet - Water Sample |
| 1 | chemical Oxygen Demand, mg/l | 9500 mg/l | 6550 mg/l |
 |
| Figure 4.14: COD value deviation of dyed outlet H2O sample |
The full dissolved solids(TDS) value of the samples was tested past times using TDS meter.
| Sl.no | Dyed outlet water | TDS (ppm) (conventional dyed H2O sample value) | TDS (ppm) (unconventional dyed H2O sample value) |
| 1 | RED HE3B | 12000 | 6000 |
| 2 | RED HE5B | 12400 | 6200 |
| 3 | YELLOW HE4R | 13000 | 6300 |
| 4 | NAVY BLUE HEGN | 12800 | 6000 |
| 5 | BLUE HEGN | 13000 | 6100 |
 |
| Figure 4.16. TDS value deviation of dyed outlet H2O sample |
CONCLUSION
In my project, the Cotton fabrics were pretreated alongside polyacrylamide, together with so reactivity of reactive dyes on fibre increased. together with so the cloth are dyed using reactive dyes without salt. The dyeing of cotton fiber alongside reactive dyes using polyacrylamide pretreated cloth inward the dye bathroom improves the dye mightiness of cellulosic fabrics alongside reactive dye, when dyeing the modified substrates.the reactive dyes tin give the axe live much to a greater extent than efficiently exhausted together with fixed onto cellulosic fabrics nether neutral weather condition inward the absence of tabular array salt .
Then Washing fastness , rubbing fastness,light fastness together with perspiration fastness of pretreated sample were amend than that for the conventionally dyed sample. The pretreated sample increases the dye uptake equally good equally deep color yield delta-E value .The full dissolved solid (TDS) content of the dyed outlet H2O efficiently reduced than conventional dyeing process. The biological oxygen demand (BOD) together with chemic oxygen demand (COD) of dyed outlet H2O likewise controlled together with reduced than conventional method dyeing. By using this pretreatment method, the next advantages were observed:
- Elimination of tabular array salt equally an electrolyte,
- Maximum fixation of dye,
- Minimum hydrolysis of dye,
- Low book of H2O requirement during the wash-off process,
- Significant savings inward physical care for costs,
- Environmentally friendly.
- Z. T. Liu, Y. Yang, L. Zhang , Z. W. Liu, together with H. Xiong (2007), "Study on the Cationic Modification together with Dyeing of Ramie Fibre", Cellulose, 14, 337-345,.
- L. Rong, together with G. Feng (20060 , Dyeing Properties of PECH-Amine Cationized Cotton alongside Acid Dyes" J. Appl. Polym. Sci., 100, 3302-3305,.
- Aravin Prince Periyasamy, Bhaarathi Dhurai, K.Thangamani (2011),Salt-free dyeing- Influenza A virus subtype H5N1 novel method of dyeing on lyocell/cotton blended fabrics alongside reactive dyes, , AUTEX Research Journal, Vol. 11, No1,.
- Mohammad Gias Uddin(2014),Study on the Performance of Eco-Alkali inward Dyeing of Cotton Fabric alongside Reactive Dyes, International Journal of Textile Science, 3(3): 51-58 DOI: 10.5923/j.textile.20140303.03
- U. Filipkowska, E. Klimiuk, S. Grabowski, E. Siedlecka(2002), Adsorption of Reactive Dyes past times Modified Chitin from Aqueous Solutions, Polish Journal of Environmental Studies Vol. 11, No. 4, 315-323.
- Mohamed Abd el-moneim Ramadan, Samar Samy, Marwa abdulhady together with Ali Ali Hebeish (20010,Eco-Friendly Pretreatment of Cellulosic Fabrics alongside Chitosan together with Its Influence on Dyeing Efficiency Textile Research Division, National Research centre, Dokki, Giza ,Egypt.
- James W. Rucker together with Darrin M. Guthrie (1998), Reduction of tabular array salt requirement inward Dyeing of cotton fiber alongside fibre alongside reactive dyes , North Carolina State University, Raleigh.
- A. Hebeish1, M. Hashem(2006),No-Salt Dyeing Behaviour of Cationized Linen Fabrics, Textile Division, National Research Centre, Dokki, Cairo, Egypt,RJTA Vol. 10 No. 2
- Awais Khatri, Rajiv Padhye, Max White (2006),Biodegradable organic salts for reactive dyeing of cotton, Keith CowlishawSchool of Fashion together with Textiles, RMIT University, Australia,https://www.researchgate.net/publication/249994609.
- Kunal , Arijit D, Dhalwinder S, Joshika, Kunal (2013),Salt Free Dyeing Design alongside Acidic (Neucleophilic Dyeing Method) together with Alkaline (Electrophilic Dyeing Method) Process on Pure Cotton Fabric past times Using Reactive Dye et al., J Textile Sci Eng 2013,, 3:3 http://dx.doi.org/10.4172/2165-8064.1000135
- H. M. Ibrahim,P together with M. M. Reda,(2015),Multi-function Modification of cotton fiber fabrics for improving utilization of reactive dyesP1PNational Research Center, Textile Research Division, Dokki, Cairo, Egypt, IJISET - International Journal of Innovative Science, Engineering & Technology, Vol. 2 Issue 7, July 2015.
- F. Shahin (2015), The Influence of Cationization on the Dyeing Performance of Cotton Fabrics alongside Direct Dyes Textile Printing, Dyeing & Finishing Dept., Faculty of Applied Arts, Helwan University, Giza, Egypt.Journal of Engineering Research together with Applications ISSN: 2248-9622, Vol. 5, Issue 8, (Part - 3) August 2015, pp.62-70.
- Hauser, P.(2000), Textile Chemist together with Colorist & American Dyestuff Reporter, vol. 32, no. 6, p. 44.
- S. L. Draper, K. R. Beck together with C. B. Smith (2002), "Characterization of the Dyeing Behavior of Cationic Cotton alongside Direct Dyes", AATCC Review, 2 (10), 24-27 .
- Y. A. Youssef (2000), " Direct Dyeing of Cotton Fabrics Pre-treated alongside Cationising Agents", Coloration Technology, 116(10), 316–322,.
- F .A. Mohamed together with E.A. El-Alfy (2013)," Improving Dyebility of Cotton Fabric via Grafting alongside Dimethylamino Ethylmethacrylate", Journal of Applied Sciences Research, 9(1), 178-183,.
- H.T. Wang together with D.M. Lewis (2002), "Chemical Modification of Cotton to Improve Fiber Dyeability", Colour. Technol., 118(4), 159-168,.
- R. Mansour, M. Farouk together with E. M. Bechir1 (2014)," Dyeing Properties of Cationized together with non Cationized Cotton Fabrics Dyed alongside Vitis vinifera L. Leaves Extract, Middle-East" Journal of Scientific Research 21 (9),1600-1604,.
- N. Ristić, I.Ristić (2012)," Cationic Modification of Cotton Fabrics together with Reactive Dyeing Characteristics", Journal of Engineered Fibers together with Fabrics, 7(4) , 113-121,.
- K. Hyde, H. Dong, together with J. P. Hinestroza (2007), " Effect of Surface Cationization on the Conformal Deposition of Polyelectrolytes over Cotton Fibers", Cellulose, 14, 615-623,.
- Chemical oxygen demand –http://www.google.co.in/search?q=chemical+oxygen+demand.pdf