Now You Know Cationization of Cotton by Using Chitosan for Reactive Dyeing to Avoid Electrolyte (Part-2)
Monday, 21 January 2019
Edit
Cationization of Cotton by Using Chitosan for Reactive Dyeing to Avoid Electrolyte (Part-2)
Md. Obydullah
Md. Shibli Sadique
Dhaka University of Engineering & Technology,
DUET, Gazipur-1700, Bangladesh
Md. Shibli Sadique
Dhaka University of Engineering & Technology,
DUET, Gazipur-1700, Bangladesh
Previous Part
2.4 Classification of Reactive Dyes:
Reactive dyes may be classified in various ways as below:
a) On the basis of reactive group:
i) Halogen (commonly chlorine) derivatives of nitrogen containing heterocycle, like 3 types-
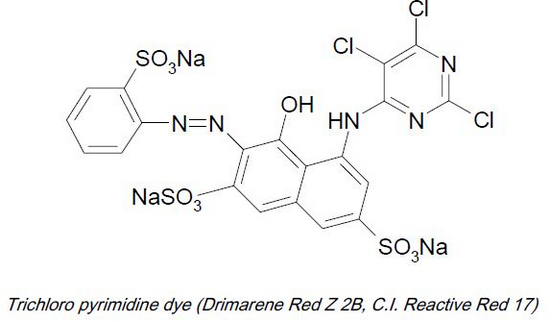
* Triazine group. e.g. Triazine derivatives: procion, cibacron.

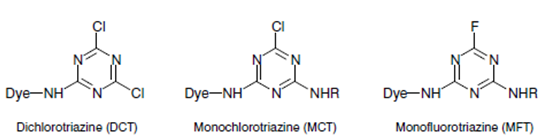 |
 |
 |
* Vinyl sulphone e.g. Vinyl sulphone: remazol
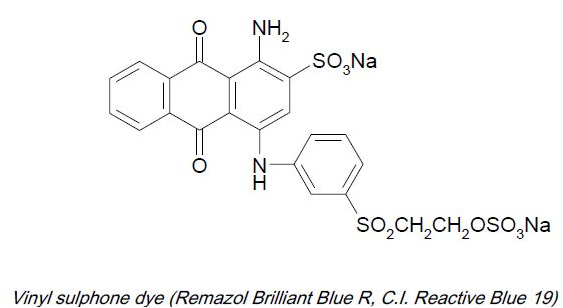
Vinyl sulphone dyestuffs possess poor affinity for cellulosic fibers in absence of salt and alkalis. For this reasons, they are suitable for use on pad. Their substantivity can be increased by addition of glauber salt or common salt and alkali, making the dyestuff suitable on all conventional dyeing machines for loose material, yarn in hanks and packages and piece goods. The dyeing methods may therefore, be classified under the padding and exhaust processes, standing baths are not recommended, as in alkaline medium inactivation of the dyestuffs by reaction with water takes place as a side reaction.
These dyes require higher dosage of alkali and salt for exhaustion and fixation. But these dyes have on continuous contact with the alkali and water medium for longer duration time dyeing process, about 30 to 40% will get hydrolyzed and become in active as dyestuff. But in order to make active and fix on the fiber we need to give higher dose of alkali and salt along with temperature of up to 60°C.
* Vinyl acrylamide e.g. Vinyl acrylamide: primazine
*Vinyl sulphonamide e.g. Vinyl sulphonamide: levafix
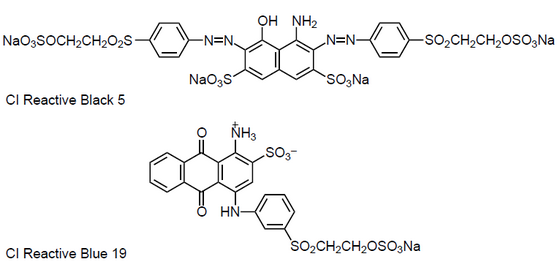 |
i) Lower reactive dye: Medium reactive dye: here pH is maintained 11-12 by using Na2CO3 in dye bath.
ii) Higher reactive dye: here pH is maintained 10-11 by using NaHCO3 in dye bath.
c) On the basis of dyeing temperature
i) Cold brand:
These types of dyes contain reactive group of high reactivity. So dyeing can be done in lower temperature i.e. 320-600C. e.g. PROCION M, LIVAFIX E.
ii) Medium brand:
This type of dyes contains reactive groups of moderate reactivity. So dyeing is done in higher temperature than that of cold brand dyes i.e. in between 600-710C temperatures e.g. Remazol, Livafix are medium brand dyes.
iii) Hot brand:
This type of dye contains reactive groups of least reactivity. So high temperature is required for dyeing i.e. 720-930 C temperature is required for dyeing. e.g. PRICION H, CIBACRON are hot brand dyes.
d) On the basis of functional group present
i) Mono functional reactive dyes.
ii) Bi functional reactive dyes.
The dyes were first introduced by Hoechst, based on ß-sulphato ethyl sulphone as a reactive group. The range made the successful breakthrough due to comprehensive shade range, versatile application, ability to be dyed at 60°C and good compatibility with other bifunctional dyes. Being a compact structure, it offers ease in wash off for removal of unfixed dyes at the end of dyeing during washing. The dye fibre bond offers good acid stability. With the limitations to above, there may be problems with reproducibility associated with it, which is not accepted especially for pale shades. There is the possibility of unlevel dyeing with a small variation in the process due to its lower molecular weight. Hetero bifunctional dyes made a get through in early 80's. The dyes were based on a quite simple but beautiful chemistry. They had filled a big vacuum of various hues of bright shades, which were not possible with vinyl sulphone type. The dyes were conventional dichlorotriazine type, with the end condensation of ß-sulphato ethyl sulphone to have different type of reactive group in a single molecule. Being a dual reactive, the primary mode of fixation is from vinyl sulphone group and the dyeing temperature is same as vinyl-sulphone, which enables good intercompatibility in between the two classes.
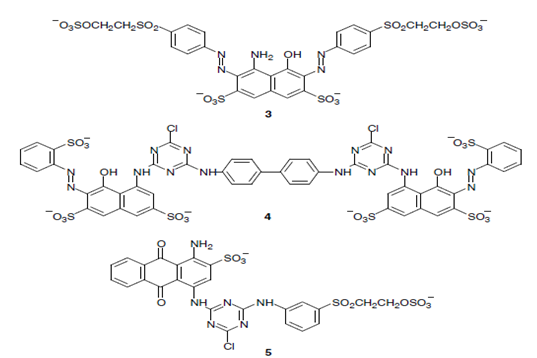 |
(4) MCT-VS type. (5) Sumifix Supra dyes (Sumito)
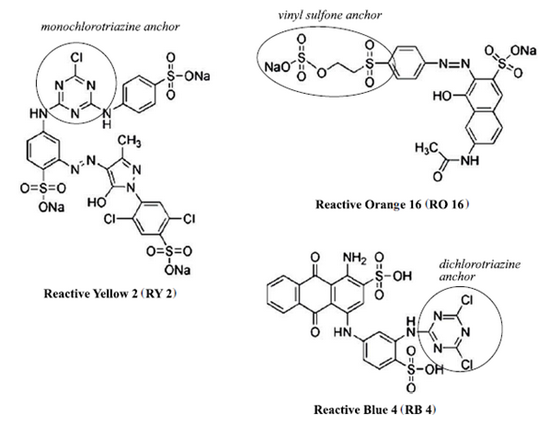 |
2.5 Reactive Dyes for Cellulosic Fibers:
Cellulosic fibers are significantly dominated by cotton. Other cellulose based fibers include viscose rayon, linen, cupraammonium rayon, jute and lyocell, and these can be dyed with dyes used on cotton. The structure of cellulosic fibers is characterized by the poly-(1, 4)-β-D-glucopyranose molecule (fig 3.2), and consequently may be considered as a polyhydric alcohol. Each glucopyranose ring on the cellulose chain contains three hydroxyl groups, a primary hydroxyl group in the 6 position and secondary hydroxyl groups in the 2 and 3 positions.
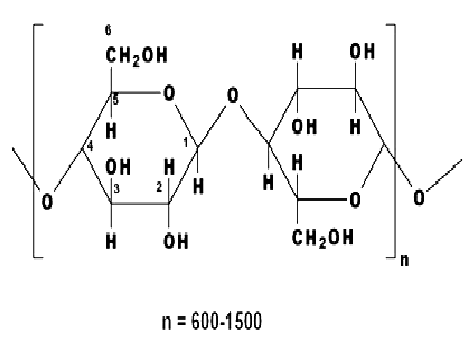 |
| Figure: 2.2 Poly-(1, 4)-β-D-glucopyranose Molecule |
Water K= 2.09x10-14
Methanol K= 8.1x10-15
Manitol K= 7.5x10-14
Cellulose K= 1.84x10-14
The dissociation constant for cellulose refers to the ionization of the hydroxyl group in the 6 position of the glucopyranose ring. The hydroxyl groups in positions 2 and 3 of the ring are less acidic. Therefore, cellulose is ionized under alkaline conditions and can behave as a nucleophile towards compounds containing electron-deficient carbon atoms (e.g. reactive dyes).
A variety of attempts have been made to bring about the formation of a covalent bond between a dye and a fiber. In this respect, there are two general approaches: producing a dye within the fiber, and producing a dye that is reactive towards the fiber. A covalent dye cellulose combination was achieved in 1895 by Cross and Bevan. These workers showed that cellulose (Cell-OH) treated with strong alkali was changed into “soda cellulose” which could be treated with benzoyl chloride to form benzoyl cellulose. The resultant benzoyl cellulose was nitrated, the nitro group was reduced, and the amino group was diazotized. The diazo group was coupled with N, N-dimethylaniline to give “dyed” fibers.
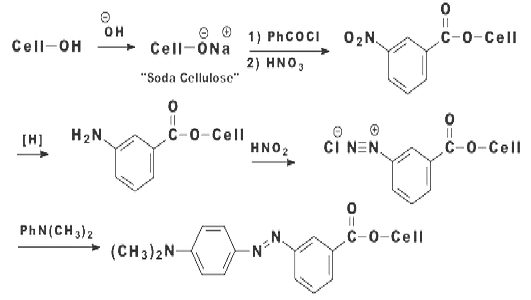 |
| Figure: 2.3 Preparation of Red Cellulose |
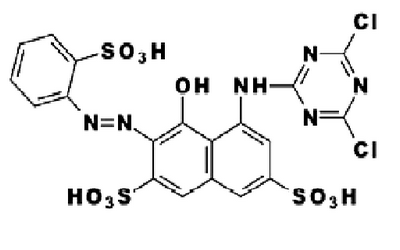 |
| Figure: 2.4 Procion Brilliant Red M-2B |
Next part will publish soon....