Now You Know Application of Ultrasound in the Preparation of Cotton and Silk Fabric (Part-1)
Friday, 8 March 2019
Edit
Application of Ultrasound in the Preparation of Cotton and Silk Fabric (Part-1)
Utpal Mondal
Govt. College of Engineering and Textile Technology
Berhampore, Murshidabad, India
Email: utpal.khs.gcettb07@gmail.com
Berhampore, Murshidabad, India
Email: utpal.khs.gcettb07@gmail.com
1. INTRODUCTION
1.1 Sound:
Sound is a form of energy, just like electricity and light. Sound is made when air molecules vibrate and move in a pattern called waves, or sound waves [1].
1.2 Ultrasound:
Sound generated above the human hearing range (20 Hz to 20 kHz) is called ultrasound. Ultrasonic vibrations travel in the form of a wave, similar to light. However, unlike light waves, which can travel in a vacuum (empty space); ultrasound requires an elastic medium such as a gas, liquid or solid. However, the frequency range normally employed in ultrasonic non-destructive testing and thickness range is 100 kHz to 50 MHz. Although ultrasound behaves in a similar manner to audible sound, it has a much shorter wavelength [2].
1.3 History:
The earliest form of an ultrasonic transducer was a whistle developed by Sir Francis Galton (1822-1911) in 1883 to investigate threshold frequency of human hearing.
The first commercial application of ultrasonic appeared around 1917 and was the first “eco-sounder” invented and developed by Paul Langévin (1872-1946). The original “echo-sounder” eventually became underwater sound navigation and ranging for submarine detection during World War 2 [3].
2. GENERAL PRINCIPLE
2.1 Introduction:
Being a sound wave, ultrasound is transmitted through any substance, solid, liquid or gas which possess elastic properties. The movement of the vibrating body is communicated to the molecules of the medium, each of which transmits the motion to an adjoining molecule before returning to approximately its original position. For liquids and gases, particle oscillation takes place in the direction of the wave and produces longitudinal waves (In a longitudinal wave the particle displacement is parallel to the direction of wave propagation) (fig.1a). Solids, however, they also possess shear elasticity, can also support tangential stresses giving rise to transverse waves (In a transverse wave the particle displacement is perpendicular to the direction of wave propagation), in which particle movement takes place perpendicular to the direction of the wave (fig. 1b) [3].
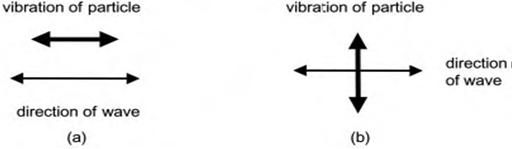 |
| Figure. 1: Wave particle movement; (a) longitudinal waves; (b) transverse waves. |
2.2 Bubble formation and the factors affecting cavitation threshold:
A bubble is a globule (small round particles) of one substance in another, usually gas in a liquid. Due to the marangoni effect (the mass transfer along an interface between two fluids due to surface tension gradient), bubbles may remain intact when they reach the surface of the immersive substance []. Cavitation is the formation of vapour cavities in liquid i.e. small liquid-cavitation-free zones (“bubbles” or “void”)-that are the consequencies of cavitational forces acting upon the cavitational liquid. It usually occurs when a liquid is subjected to rapid changes of pressure that cause the formation of cavities where the pressure is relatively low [4].
The effects are as follows:
2.2.1 Effect of gas and particulate matter:
The progression of a sound wave through a liquid medium caused the molecules to oscillate about their mean position. During the compression cycle, the average distance between the molecules decreased, whilst during rarefaction the distances increased. If a sufficiently large negative pressure is applied to the liquid, such that the average distance between the molecules exceeds the critical molecular distance necessary to hold the liquid intact, the liquid will break down and voids or cavities will be created i.e. cavitation bubbles will be formed (cavitation is the formation of gas bubbles of a flowing liquid in a region where the pressure of the liquid falls below its vapour pressure. Cavitation is usually divided into two classes of behaviour: inertial cavitation, and non-inertial cavitation). Once produced these cavities, voids or bubbles, may grow in size until the maximum of the negative pressure has been reached.
 |
| Figure. 2: Erosion of (a) initial surface; (b) eroded surface |
To create bubble in water, provide the maximum rarefaction. Thus there will probably be several different types of cavity in the liquid:
- The empty cavity (true cavitation),
- The vapour filled cavity,
- The gas filled cavity, unless the liquid is totally degassed, and
- A combination of vapour and gas filled cavities [3].
Since it is necessary for the negative pressure in the rarefaction cycle to overcome the natural cohesive forces acting in the liquid, any increase in these forces will increase the threshold of cavitation. One method of increasing these forces is to increase the viscosity of the liquid.
The effect, though not insignificant, is hardly dramatic. Taking corn and castor oils as examples, a ten-fold increase in viscosity has only led to a 30% increase in the acoustic pressure needed bring about cavitation [3].
2.2.3 Effects of applied frequency:
To completely rupture a liquid and hence provide a void, which may subsequently become filled with gas or vapour, requires a finite time. For sound waves with high frequencies, the time required to create the bubble may be longer than available during the rarefaction cycle. Thus it might anticipated that as the frequency increases the production of cavitation bubbles become more difficult to achieve in the available time and the greater sound intensities will need to be employed, over these shorter periods, to ensure that the cohesive forces of the liquid are overcome.
 |
| Figure. 3: Variation in threshold intensity with frequency; (a) aerated water; (b) air free water [3] |
The final factor to be considered here, and known to affect the cavitation threshold, is the temperature. In general the threshold limit has been found to increase with decrease in temperature. This may in part be due to increases in either the surface tension or viscosity of the liquid as the temperature decreases, or it may be due to the decreases in liquid vapour pressure [3].