Now You Know Cationization of Cotton by Using Chitosan for Reactive Dyeing to Avoid Electrolyte (Part-3)
Monday, 21 January 2019
Edit
Cationization of Cotton by Using Chitosan for Reactive Dyeing to Avoid Electrolyte (Part-3)
Md. Obydullah
Md. Shibli Sadique
Dhaka University of Engineering & Technology,
DUET, Gazipur-1700, Bangladesh
Md. Shibli Sadique
Dhaka University of Engineering & Technology,
DUET, Gazipur-1700, Bangladesh
Previous Part
2.6 Chemical Reactions between Reactive Dyes and Fibers:
a) Nucleophilic Substitution:
Nucleophilic substitution characterizes dye-fiber fixation that occurs when a leaving group in the reactive system is displaced as a result of an interaction with a nucleophilic group on the polymer chain. Nucleophilic substitution is facilitated by the electron withdrawing properties of the aromatic nitrogens, and the chlorine, and the anionic intermediate is resonance stabilised as well. This resonance means that the negative charge is delocalised onto the electronegative nitrogens.
For example:


The major fibre-reactive group which reacts this way contains six-membered, heterocyclic, aromatic rings, with halogen substituents.
For example, the Procion dye-
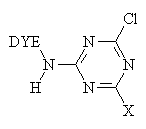
The reaction of a monochlorotriazine reactive dye with a hydroxy group of cellulose is typical of this process.
a)

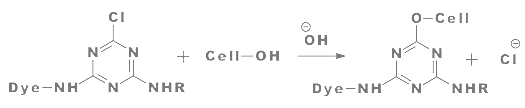 |
| Figure: 2.5 Reaction of a Monochlorotriazine Dye with Cellulose |
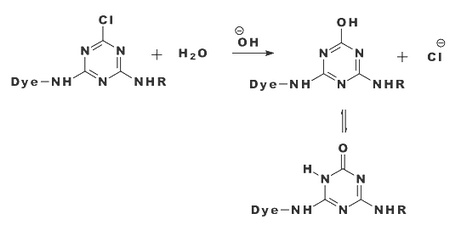 |
| Figure: 2.6 Hydrolysis of a Monochlorotriazine Dye |
Nucleophilic addition characterizes the dye-fiber reaction in which a nucleophilic group in the fiber adds across an activated carbon-carbon double bond in the reactive group. Most of reactive systems used contain a vinylsulphone moiety. The vinylsulphone reactive group itself is usually not present in commercial form of the dyes employed. Instead, more stable precursor such as the β-sulphatoethylsulphone group is used. The two-stagenprocess associated with fiber fixation is structurally related dyes containing a β-sulphatoethylsulphamoyl group probably form a cyclic compound capable of reacting with cellulose to give cellulose ether.
Systems based on activated double bonds also undergo a competitive hydrolysis reaction.
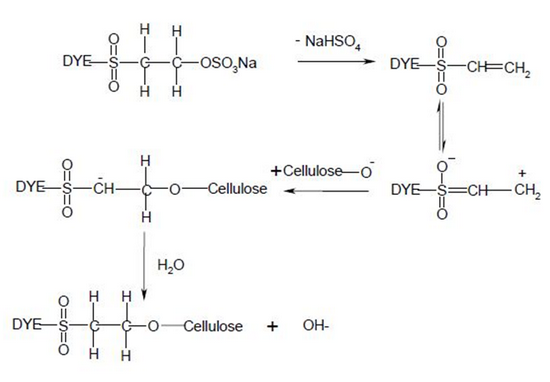 |
| Figure: 2.7 Nucleophilic Addition Involving to a Vinylsulphone Dye and Cellulose. |
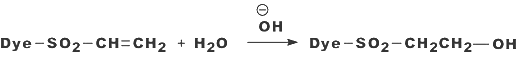 |
| Figure: 2.8 Reaction of Water with a Vinylsulphone Dye |
CHAPTER 3
COTTON FIBER
COTTON FIBER
3.1 Cotton Fibre
Cotton is unique in nature which has the highest percentage of cellulose. It varies from 90% to 95%. This concentration depends upon many factors, such that maturity level and variety of cotton, place on cottonseed from where it is drawn. In cotton fiber there is certain non-cellulosic matter, which plays important role in growth and on forth coming processes. These are located either on the surface of the fiber or in center of fiber means inside the lumen. Being a natural fiber, there are many factors which affect the structure of cotton cellulose. There are primary and secondary walls in the cotton cellulose. Primary wall or outer wall is less crystalline and has less percentage of cellulose. Whereas, secondary wall is around the lumen and consist of pure cellulose cotton fiber is composed of the crystalline and amorphous structure.
3.2 Chemical Structure of Cotton Fiber
The molecular structure of cellulose has always been of great interest to scientists and over time several structures have been proposed. The linear polymer, β-D-glucopyronose with 1,4-glycosidic bonds, is the widely accepted structure for cellulose. Consequently it may be considered as a polyhydric alcohol. Each glucopyranose ring in the cellulose chain contains three hydroxyl groups, a primary hydroxyl group in the 6- position and secondary hydroxyl groups in the 2- and 3- positions.
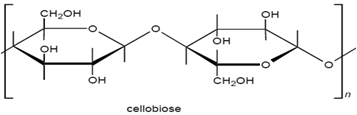 |
| Figure: 3.1 Cellubiose Unit. |
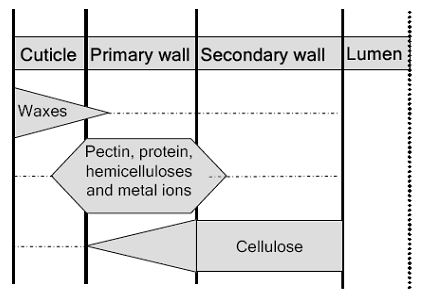
The basic ingredients, that are responsible for complicated interconnections in the primary wall, are cellulose, hemicelluloses, pectins, proteins and ions. These components are present throughout the primary wall. The only difference is the concentration and nature of each component, as when approaching the secondary wall. In the secondary wall, only crystalline cellulose is present, which is highly ordered and has compact structure the cellulose fibrils in the secondary wall are laying parallel to one another.
Table: 3.1 Typical Composition of Dry Mature Cotton Fiber
| Constituents | Composition (%) | |
| Whole fiber | Outer layer | |
| Cellulose | 94 | 54 |
| Waxes | 0.6-1.3 | 14 |
| Pectin substances | 0.9-1.2 | 9 |
| Protein (nitrogen substances) | 0.6-1.3 | 8 |
| Ash | 1.2 | 3 |
| Organic Acid | 0.8 | - |
| Others | 1.4 | 12 |
a. Cellulose: 94%. It is main part of the cotton fiber and secondary wall posse’s highest percentage of the total cellulose
b. Waxes: 0.6-1.3%. It is higher monovalent alcohol-tractional, palmitic, oleic acid, glycerin. Its melting point is 77.0. It is found on surface and in primary wall.
c. Pectin’s: 0.9 -1.2%. These are ploygalacturonic acid, and its magnesium salts, methyl ester, xylose. These are mainly present in primary wall.
d. Proteins: 0.6-1.3%. These are protoplasm rest in lumen and aspartic, glutamic acid and proline, 0.2-0.3% of nitrogen are found in primary wall
e. Ash: 1.2%
f. Organic acids: 0.5-1.00%. Salts of citric and L-maleic acid
g. Others: (i) Mineral salts: 0.7-1.6%. Hypochlorites of sulphates, phosphates, oxides of silicon, calcium, potassium, magnesium. (ii) Sugar: 0.3%. Glucose, galactose, fructose, pentose. (iii) Toxine: 0.9%. Endotoxine, evolved from bacterial cells.
3.4 Fiber Macro Structure
Under a microscope, a cotton fiber appears as a very fine, regular fiber. It ranges in length from about 10mm to 65 mm, depending upon the quality of the fiber. Each cotton fiber is composed of concentric layers. The cuticle layer on the fiber itself is separable from the fiber and consists of wax and pectin materials. The primary wall, the most peripheral layer of the fiber, is composed of cellulosic crystalline fibrils. The secondary wall of the fiber consists of three distinct layers. All three layers of the secondary wall include closely packed parallel fibrils with spiral winding of 25-35o and represent the majority of cellulose within the fiber. The innermost part of cotton fiber- the lumen- is composed of the remains of the cell contents. Before boll opening, the lumen is filled with liquid containing the cell nucleus and protoplasm. The twists and convolutions of the dried fiber are due to the removal of this liquid. The cross section of the fiber is bean-shaped, swelling almost round when moisture absorption takes place.
The overall contents are broken down into the following components.
3.5 Polymer System of Cotton
The density method used to determine cellulose Crystallinity is based on the density gradient column, where two solvents of different densities are partially mixed. Degree of Crystallinity is, then, determined from the density of the sample, while densities of crystalline and amorphous cellulose forms are known (1.505 and 1.556 respectively). Orientation of untreated cotton fiber is poor because the crystallites are contained in the micro fibrils of the secondary wall, oriented in the steep spiral (25-30o) to the fiber axis.
3.6 Chemistry of Cotton Cellulose
Cellulose is an insoluble substance and mainly composed of polysaccharide, which holds chains of glucose monomers. It is only soluble in some specific solvents. It is the main constituent of plant cell walls and as well as of vegetable fibers. Cotton is one of the vegetable fibers which have the highest percentage of cellulose. There is a diverse structures and compositions of cellulose of cotton. Its structure makes it divergent from other naturally occurring matters. Cotton cellulose is unique in many ways and possesses distinct characteristics which make it highly useful for many purposes. It has been assumed that cotton cellulose structure is based on glucose unit only. However, it is also believed that there is modest amount of pentose is present, which is removed during scouring process. They further report that it has been notices from the chromatographic analysis that glucose, xylose, arabinose and a trace of rhamnose are also present in raw cotton. There is no evidence of the presence of pentose. Cotton cellulose is highly crystalline in nature and well oriented and has along and rigid molecular structure. The 1,4-D glucopyranose are the principle building blocks of cotton cellulose chain and are linked by l,4-glucodic bonds. Free rotation of the anhydrogluco-pyranose C-O-C link is stopped by steric effects. There are three hydroxyl groups attached to each anhydroglucose. One group is attached at C-6 and two at C-2 and C-3. Due to the presence of hydroxyl groups and the chain conformation, there are many more bonds possible (inter molecule and intramolecular). Such bonds make the fiber more rigid by increasing the rigidity of the structure of cotton cellulose.
3.7 Cross Section of Cotton Fiber
The cell wall is a dynamic structure which composition and form can change markedly, not only during cell growth but also after the cells have become matured. The cotton fiber is structurally built up into concentric zones and a hollow central core known as the lumen.
The mature fiber essentially consists of (from outside to inside) - the cuticle i.e. the outermost layer, the primary cell wall, the secondary wall and the lumen. Figure 1.1 systematically shows the different layers present in the cotton fiber with the compositions of each layer. Cotton contains nearly 90% of cellulose and around 10% of non-cellulosic substances, which are mainly located in the cuticle and primary wall of the fiber. Typical components in dry mature cotton fibers most of the non-cellulosic materials are present in the outer layers of cotton fiber.
Figure illustrates schematically the distribution of cellulose and other non-cellulosic materials in the various layers of cotton fiber.. The outermost layer is the cuticle. It is a thin film of mostly fats and waxes. Owing to non-structured orientation of cellulose and non-cellulosic materials, the primary wall surfaces is unorganized and open. This gives the flexibility to the primary wall, which is required during the cell growth.
3.9 Different Layer of Cotton Cellulose Structure
a. Cuticle – the outermost layer
The aerial surfaces of vascular plants are covered with an extra cellular layer called the cuticle or cuticular membrane that overlays the cell wall of epidermal cells. The term “cotton waxes” has been used for all lipid compounds found in the cuticle of fiber. The main function ascribed to the cuticle is to minimize water losses from cotton fiber. Other functions are, to limit the loss of substances from fibers internal tissues, and to protect the fiber against physical, chemical and biological aggressions. The cuticle contains primary alcohols, higher fatty acids, hydrocarbons, aldehydes, glycerides, sterols, acyl components, resins, cutin and sobering, which are called waxes. The cuticle gives a soft touch to the fiber and reduces the friction forces during spinning. At the same time, presence of these waxy materials is Initiation of fiber growth detrimental in chemical processing of cotton yarn because it gives the fiber surface a high hydrophobicity. The cotton waxes are solid substances with rather high and wide range of melting points (64°C to 214°C) and constitute around 0.4 to 0.8% dry weight of raw cotton After treatment with boiling NaOH, waxes are hydrolyzed into a sodium salt of the fatty acid and alcohol. Apart from the above mentioned components in the cuticle, there are also some complex biopolymers present.
b. The primary wall
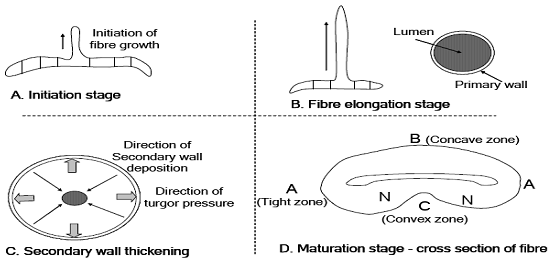
Cotton is longest single cell as compared to other agro-based fibers. Its growth initiates with the single cell from the individual epidermal cells on the outer integument of the ovules in the cotton fruit. There is a number of cotton fibers grow simultaneously from the same cotton bowl (cotton seed). Each fiber presents a single cell of plant. In first step this plant goes to its full length and during this period primary wall is formed. This primary wall holds the whole cell plant and keep it aligns and well oriented. During this period, when the fiber is totally consisting of primary walls, fiber width is 2.98 mm and crystalline percentage is up to 30%.
The primary wall in a cotton fiber is a thin film with a thickness of about 0.5 μm. It serves as the exterior surface of the fiber. In the primary wall, apart from amorphous cellulose, most of the constituents are non-cellulosic materials. This layer is flexible and swells uniformly in all directions. the typical composition of the primary wall with details of each component. The primary wall contains amorphous cellulose, pectin’s, proteins, hemicelluloses and coloring components. It is important to study the individual component and interconnections in the primary wall, for attacking specific components with enzymes to destabilize the primary wall efficiently.
A brief description of the primary wall components, followed by a description of their interconnections is given below.
Details of each constituent in the primary wall
i) Cellulose:
Cellulose in the primary wall is heterogeneous and has a low degree of polymerization (DP up to 2000 glucose units) compared with cellulose in the secondary wall. The amorphous region of cellulose in a cotton fiber is characterized by its ability to swell in water. The orientation of cellulose macromolecules in the primary wall is low, that means that individual macromolecules are not arranged in any definite order. The cellulose micro fibrils in the primary wall are surrounded by a matrix of other non-celluloses. Micro fibrils of cellulose are crystalline aggregates of β(1-4)-linked glucose polymers. They are omnipresent elements of the plant cell wall and are responsible for much of its tensile strength.
ii) Hemicelluloses:
Hemi cellulose is the name of a heterogeneous group of branched matrix forming polysaccharides. Hemicelluloses bind non-covalently to the surface of cellulose micro fibrils in the primary wall. They form a coating over the cellulose micro fibrils and are able to cross-link them into a complex network of the primary wall. There are several classes of hemicelluloses with an average 50 glucose units that are linearly β (1-4)-linked to one another. The difference between various classes of hemicelluloses is expressed in terms of oligosaccharide side chains
iii) Glycoprotein:
Glycoprotein also known as extensions account up to 15% of the primary cell wall mass. Glycoprotein contains a protein backbone with extended rod like carbohydrates that protrude outwards. The carbohydrates in the glycoprotein account for 65% of the total structure. For a cotton fiber, these rod shape extensions are made up of roughly 300 amino acids, and abundantly contain hydroxyl-proline (Hyp). Most of the hydroxyl-prolines are glycosylated with chains of three or four sugar residues e.g. arabinose and galactose. The carboxyl-terminal peptides in the glycoprotein molecule are covalently linked by disulfide bonds and often contain an oligosaccharide chain. These oligosaccharides chains are also interconnected with other polysaccharides in the primary wall.
iv) Pectin’s:
Pectin’s are acidic polysaccharides, which are found in fruits, fibers and vegetables. Pectin being a non-cellulosic material in cotton fibers plays several important roles. It contributes to the firmness and structure of cotton fiber, both as a part of the primary cell wall. Pectin acts as cementing material for the cellulosic network in the primary wall. Pectin, as a hydrating agent, controls the movement of water and other plant fluids through the rapidly growing fiber.
v) Coloring matters:
Coloring matter (pigments) in cotton fibers is rarely studied. In the natural state, cotton is off-white, cream, brownish or greyish green, depending on the source and growing conditions. However these coloring components do not take part in the scouring process.
vi) Metal contents:
The primary wall of a cotton fiber contains different quantities of metal depending on their growing conditions and source. Potassium is the most abundant metal ion in cotton fibers followed by magnesium and calcium. Other metal ions which are present in traces are sodium, iron, manganese, copper and zinc. Removal of calcium is essential during the scouring process for better primary wall destabilization. However, removal of rest of the metals from cotton fibers is also important, because they can contribute to problems during further wet-pretreatment processes like oxidative bleaching.
c. The secondary wall
Secondary wall formation originates just after the completion of primary wall. Primary wall takes around 20-25 days and after that secondary wall synthesis commences which takes 15-22 days and it continues for 30-40days. During this period fiber gains weigh of 130 ng/mm, as compared to 2ng/mm while primary wall was under development. After completion of primary wall, secondary wall is formed. Secondary wall is in the shape of a ring inside the tube or cylinder. Notwithstanding, during this period a lumen in the center is also produced at the time of fiber maturity. Secondary wall is an example of pure cellulose. Study further reveals that secondary wall is 94% of the total mass of the fiber material. Thereby secondary wall is contemplated as the main responsible for all sorts of mechanical properties. There is a slight variation in the density of the cell wall before and after desiccation. It is 1.55 gm before drying and after dehydration it reduces to 1.52 gm under the 65% RH conditions.
Cellulose in the secondary wall is characterized by a higher degree of polymerization (nearly 5000 units) compared with cellulose in the primary wall. Table 2.4 shows the various units and sub-units of cotton cellulose with their diameter. In the secondary wall of a cotton fiber, two cellulose molecules can form a long planar chain of β (1-4)-linked glucose units, resulting in a ribbon like structure. These two cellulose chains forms a sheet, which is called an elementary fibril. Micro fibrils of cellulose are crystalline aggregates of approximately 21 elementary cells (Table 2.4). Intermolecular hydrogen bonds play an important role to stabilize chains of elementary fibril to become a micro fibril. Finally, a large numbers of such micro fibrils are laying in parallel direction forms the various layers of the secondary cell wall.
b. Waxes: 0.6-1.3%. It is higher monovalent alcohol-tractional, palmitic, oleic acid, glycerin. Its melting point is 77.0. It is found on surface and in primary wall.
c. Pectin’s: 0.9 -1.2%. These are ploygalacturonic acid, and its magnesium salts, methyl ester, xylose. These are mainly present in primary wall.
d. Proteins: 0.6-1.3%. These are protoplasm rest in lumen and aspartic, glutamic acid and proline, 0.2-0.3% of nitrogen are found in primary wall
e. Ash: 1.2%
f. Organic acids: 0.5-1.00%. Salts of citric and L-maleic acid
g. Others: (i) Mineral salts: 0.7-1.6%. Hypochlorites of sulphates, phosphates, oxides of silicon, calcium, potassium, magnesium. (ii) Sugar: 0.3%. Glucose, galactose, fructose, pentose. (iii) Toxine: 0.9%. Endotoxine, evolved from bacterial cells.
3.4 Fiber Macro Structure
Under a microscope, a cotton fiber appears as a very fine, regular fiber. It ranges in length from about 10mm to 65 mm, depending upon the quality of the fiber. Each cotton fiber is composed of concentric layers. The cuticle layer on the fiber itself is separable from the fiber and consists of wax and pectin materials. The primary wall, the most peripheral layer of the fiber, is composed of cellulosic crystalline fibrils. The secondary wall of the fiber consists of three distinct layers. All three layers of the secondary wall include closely packed parallel fibrils with spiral winding of 25-35o and represent the majority of cellulose within the fiber. The innermost part of cotton fiber- the lumen- is composed of the remains of the cell contents. Before boll opening, the lumen is filled with liquid containing the cell nucleus and protoplasm. The twists and convolutions of the dried fiber are due to the removal of this liquid. The cross section of the fiber is bean-shaped, swelling almost round when moisture absorption takes place.
The overall contents are broken down into the following components.
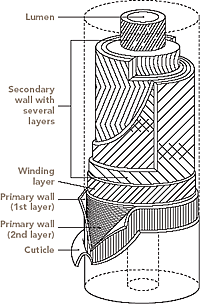 |
| Figure: 3.2 Fiber Macro Structure |
The density method used to determine cellulose Crystallinity is based on the density gradient column, where two solvents of different densities are partially mixed. Degree of Crystallinity is, then, determined from the density of the sample, while densities of crystalline and amorphous cellulose forms are known (1.505 and 1.556 respectively). Orientation of untreated cotton fiber is poor because the crystallites are contained in the micro fibrils of the secondary wall, oriented in the steep spiral (25-30o) to the fiber axis.
3.6 Chemistry of Cotton Cellulose
Cellulose is an insoluble substance and mainly composed of polysaccharide, which holds chains of glucose monomers. It is only soluble in some specific solvents. It is the main constituent of plant cell walls and as well as of vegetable fibers. Cotton is one of the vegetable fibers which have the highest percentage of cellulose. There is a diverse structures and compositions of cellulose of cotton. Its structure makes it divergent from other naturally occurring matters. Cotton cellulose is unique in many ways and possesses distinct characteristics which make it highly useful for many purposes. It has been assumed that cotton cellulose structure is based on glucose unit only. However, it is also believed that there is modest amount of pentose is present, which is removed during scouring process. They further report that it has been notices from the chromatographic analysis that glucose, xylose, arabinose and a trace of rhamnose are also present in raw cotton. There is no evidence of the presence of pentose. Cotton cellulose is highly crystalline in nature and well oriented and has along and rigid molecular structure. The 1,4-D glucopyranose are the principle building blocks of cotton cellulose chain and are linked by l,4-glucodic bonds. Free rotation of the anhydrogluco-pyranose C-O-C link is stopped by steric effects. There are three hydroxyl groups attached to each anhydroglucose. One group is attached at C-6 and two at C-2 and C-3. Due to the presence of hydroxyl groups and the chain conformation, there are many more bonds possible (inter molecule and intramolecular). Such bonds make the fiber more rigid by increasing the rigidity of the structure of cotton cellulose.
3.7 Cross Section of Cotton Fiber
The cell wall is a dynamic structure which composition and form can change markedly, not only during cell growth but also after the cells have become matured. The cotton fiber is structurally built up into concentric zones and a hollow central core known as the lumen.
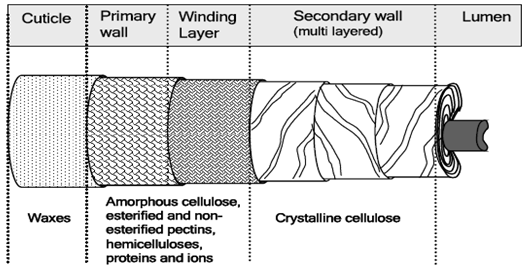 |
| Figure: 3.3 A schematic Representation of Mature Cotton Fiber Showing its Various Layers. |
Figure illustrates schematically the distribution of cellulose and other non-cellulosic materials in the various layers of cotton fiber.. The outermost layer is the cuticle. It is a thin film of mostly fats and waxes. Owing to non-structured orientation of cellulose and non-cellulosic materials, the primary wall surfaces is unorganized and open. This gives the flexibility to the primary wall, which is required during the cell growth.
3.9 Different Layer of Cotton Cellulose Structure
 |
| Figure: 3.4 A Schematic Representation of the Cellulosic Materials in the Cotton Fiber. |
The aerial surfaces of vascular plants are covered with an extra cellular layer called the cuticle or cuticular membrane that overlays the cell wall of epidermal cells. The term “cotton waxes” has been used for all lipid compounds found in the cuticle of fiber. The main function ascribed to the cuticle is to minimize water losses from cotton fiber. Other functions are, to limit the loss of substances from fibers internal tissues, and to protect the fiber against physical, chemical and biological aggressions. The cuticle contains primary alcohols, higher fatty acids, hydrocarbons, aldehydes, glycerides, sterols, acyl components, resins, cutin and sobering, which are called waxes. The cuticle gives a soft touch to the fiber and reduces the friction forces during spinning. At the same time, presence of these waxy materials is Initiation of fiber growth detrimental in chemical processing of cotton yarn because it gives the fiber surface a high hydrophobicity. The cotton waxes are solid substances with rather high and wide range of melting points (64°C to 214°C) and constitute around 0.4 to 0.8% dry weight of raw cotton After treatment with boiling NaOH, waxes are hydrolyzed into a sodium salt of the fatty acid and alcohol. Apart from the above mentioned components in the cuticle, there are also some complex biopolymers present.
b. The primary wall
Cotton is longest single cell as compared to other agro-based fibers. Its growth initiates with the single cell from the individual epidermal cells on the outer integument of the ovules in the cotton fruit. There is a number of cotton fibers grow simultaneously from the same cotton bowl (cotton seed). Each fiber presents a single cell of plant. In first step this plant goes to its full length and during this period primary wall is formed. This primary wall holds the whole cell plant and keep it aligns and well oriented. During this period, when the fiber is totally consisting of primary walls, fiber width is 2.98 mm and crystalline percentage is up to 30%.
The primary wall in a cotton fiber is a thin film with a thickness of about 0.5 μm. It serves as the exterior surface of the fiber. In the primary wall, apart from amorphous cellulose, most of the constituents are non-cellulosic materials. This layer is flexible and swells uniformly in all directions. the typical composition of the primary wall with details of each component. The primary wall contains amorphous cellulose, pectin’s, proteins, hemicelluloses and coloring components. It is important to study the individual component and interconnections in the primary wall, for attacking specific components with enzymes to destabilize the primary wall efficiently.
A brief description of the primary wall components, followed by a description of their interconnections is given below.
Details of each constituent in the primary wall
i) Cellulose:
Cellulose in the primary wall is heterogeneous and has a low degree of polymerization (DP up to 2000 glucose units) compared with cellulose in the secondary wall. The amorphous region of cellulose in a cotton fiber is characterized by its ability to swell in water. The orientation of cellulose macromolecules in the primary wall is low, that means that individual macromolecules are not arranged in any definite order. The cellulose micro fibrils in the primary wall are surrounded by a matrix of other non-celluloses. Micro fibrils of cellulose are crystalline aggregates of β(1-4)-linked glucose polymers. They are omnipresent elements of the plant cell wall and are responsible for much of its tensile strength.
ii) Hemicelluloses:
Hemi cellulose is the name of a heterogeneous group of branched matrix forming polysaccharides. Hemicelluloses bind non-covalently to the surface of cellulose micro fibrils in the primary wall. They form a coating over the cellulose micro fibrils and are able to cross-link them into a complex network of the primary wall. There are several classes of hemicelluloses with an average 50 glucose units that are linearly β (1-4)-linked to one another. The difference between various classes of hemicelluloses is expressed in terms of oligosaccharide side chains
iii) Glycoprotein:
Glycoprotein also known as extensions account up to 15% of the primary cell wall mass. Glycoprotein contains a protein backbone with extended rod like carbohydrates that protrude outwards. The carbohydrates in the glycoprotein account for 65% of the total structure. For a cotton fiber, these rod shape extensions are made up of roughly 300 amino acids, and abundantly contain hydroxyl-proline (Hyp). Most of the hydroxyl-prolines are glycosylated with chains of three or four sugar residues e.g. arabinose and galactose. The carboxyl-terminal peptides in the glycoprotein molecule are covalently linked by disulfide bonds and often contain an oligosaccharide chain. These oligosaccharides chains are also interconnected with other polysaccharides in the primary wall.
iv) Pectin’s:
Pectin’s are acidic polysaccharides, which are found in fruits, fibers and vegetables. Pectin being a non-cellulosic material in cotton fibers plays several important roles. It contributes to the firmness and structure of cotton fiber, both as a part of the primary cell wall. Pectin acts as cementing material for the cellulosic network in the primary wall. Pectin, as a hydrating agent, controls the movement of water and other plant fluids through the rapidly growing fiber.
v) Coloring matters:
Coloring matter (pigments) in cotton fibers is rarely studied. In the natural state, cotton is off-white, cream, brownish or greyish green, depending on the source and growing conditions. However these coloring components do not take part in the scouring process.
vi) Metal contents:
The primary wall of a cotton fiber contains different quantities of metal depending on their growing conditions and source. Potassium is the most abundant metal ion in cotton fibers followed by magnesium and calcium. Other metal ions which are present in traces are sodium, iron, manganese, copper and zinc. Removal of calcium is essential during the scouring process for better primary wall destabilization. However, removal of rest of the metals from cotton fibers is also important, because they can contribute to problems during further wet-pretreatment processes like oxidative bleaching.
c. The secondary wall
Secondary wall formation originates just after the completion of primary wall. Primary wall takes around 20-25 days and after that secondary wall synthesis commences which takes 15-22 days and it continues for 30-40days. During this period fiber gains weigh of 130 ng/mm, as compared to 2ng/mm while primary wall was under development. After completion of primary wall, secondary wall is formed. Secondary wall is in the shape of a ring inside the tube or cylinder. Notwithstanding, during this period a lumen in the center is also produced at the time of fiber maturity. Secondary wall is an example of pure cellulose. Study further reveals that secondary wall is 94% of the total mass of the fiber material. Thereby secondary wall is contemplated as the main responsible for all sorts of mechanical properties. There is a slight variation in the density of the cell wall before and after desiccation. It is 1.55 gm before drying and after dehydration it reduces to 1.52 gm under the 65% RH conditions.
Cellulose in the secondary wall is characterized by a higher degree of polymerization (nearly 5000 units) compared with cellulose in the primary wall. Table 2.4 shows the various units and sub-units of cotton cellulose with their diameter. In the secondary wall of a cotton fiber, two cellulose molecules can form a long planar chain of β (1-4)-linked glucose units, resulting in a ribbon like structure. These two cellulose chains forms a sheet, which is called an elementary fibril. Micro fibrils of cellulose are crystalline aggregates of approximately 21 elementary cells (Table 2.4). Intermolecular hydrogen bonds play an important role to stabilize chains of elementary fibril to become a micro fibril. Finally, a large numbers of such micro fibrils are laying in parallel direction forms the various layers of the secondary cell wall.
 |
| Figure: 3.5 An illustration of various stages of cotton fiber growth. |
Next part will publish soon.....