Yaa Dyeing Of Cotton Wool Textile Amongst Optical Brightening Agent (Oba)
Wednesday, 19 December 2018
Edit
Dyeing of Cotton Fabric alongside Optical Brightening Agent (OBA)
Mustaque Ahammed Mamun
Department of Textile Engineering
Dhaka University of Engineering & Technology (DUET)
Cell: +8801723300703
Email: mamuntex09@gmail.com
Department of Textile Engineering
Dhaka University of Engineering & Technology (DUET)
Cell: +8801723300703
Email: mamuntex09@gmail.com
Theory:
Optical Brightening Agent (OBA) is a colorless or real pale xanthous colored organic compound. When added onto a cotton fiber cloth hence increases the apparent reflectance into visible share past times absorbing ultra-violet radiations together with hence converting it into visible region. Thus increment the whiteness or brightness.
When OBA absorb a photon of lite an electron is raised from the dry reason acre (S0) of the molecule to i of its activated singlet states ( S1,S2,…..). Transitions from a singlet to a triple acre are quantum mechanically. Absorption occurs when the molecule is inward its dry reason state, the vibration marking of activated acre reached past times absorption existence decided past times the size of the quantum of liberate energy E.
Electron has vogue backwards dry reason acre & Vibrating liberate energy volition hold upwards lost earlier fluorescence occurs.
Simultaneous the purpose of photon out every bit oestrus energy.
Objectives
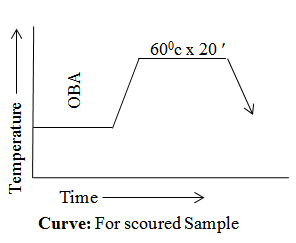
For Scoured Sample
For Scoured Sample
OBA = Sample Weight x Recipe %
= 8 x 10/100
= 0. 8 gm
Water = 8 x xxx = 240 ml
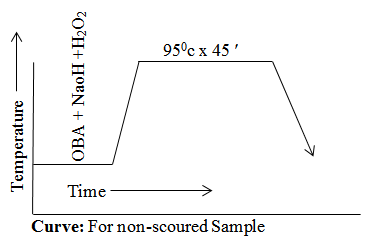
For Non-scoured Sample
OBA = Sample Weight x Recipe %
= v x 10/100
= 0. v gm
NaOH = Total liquor x Recipe Amount (gm/L)
= 250 x 3/100
= 0. 75 gm
H2O2 = Total liquor x Recipe Amount (gm/L)
= 250 x 4/100
= 1 gm
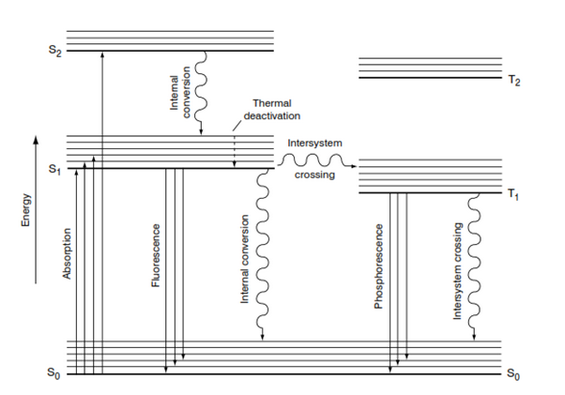 |
| Fig: Absorption & Fluorescence Process |
Electron has vogue backwards dry reason acre & Vibrating liberate energy volition hold upwards lost earlier fluorescence occurs.
Simultaneous the purpose of photon out every bit oestrus energy.
Objectives
- To know the application of OBA on cotton fiber fabric.
- To know the lawsuit of OBA on cotton fiber fabric.
- To know the procedure sequence of OBA application.
- To know the theory of OBA.
- Beaker.
- Pipette.
- Mug.
- Gas burner.
- Graduate Cylinder.
- Thermometer.
- Stirrer.
- Cotton fabric.
- OBA.
- Chemicals
For Scoured Sample
- Sample Weight ------------------------ 8 gm
- OBA ------------------------------------ 10%
- Time x Temperature -------------------- 600c x 20′
- M : L -------------------------------------- 1:30
- Sample Weight -------------------------- v gm
- OBA ------------------------------------- 10%
- NaoH -------------------------------------- three gm/L
- H2O2 ------------------------------------------------------- iv gm/L
- Time x Temperature --------------------- 950c x 45 ′
- M : L -------------------------------------- 1 : fifty
For Scoured Sample
OBA = Sample Weight x Recipe %
= 8 x 10/100
= 0. 8 gm
Water = 8 x xxx = 240 ml
For Non-scoured Sample
OBA = Sample Weight x Recipe %
= v x 10/100
= 0. v gm
NaOH = Total liquor x Recipe Amount (gm/L)
= 250 x 3/100
= 0. 75 gm
H2O2 = Total liquor x Recipe Amount (gm/L)
= 250 x 4/100
= 1 gm
Water = v x fifty = 250 ml
Curve
 Result & give-and-take
Result & give-and-take
From 2 sample, ( i sample is scoured earlier applied OBA & closed to other sample is Same can scouring & application of OBA) nosotros tin give notice say that, which sample accept been scoured earlier application of OBA is less brilliant than the sample which is same can scouring & application of OBA. Because on which sample exclusively applied OBA this is scoured earlier closed to days. Also 2 sample are to a greater extent than white than greyish cloth or scoured fabric.
Comments & Conclusion
From this experiment nosotros known nearly OBA together with the application procedure of OBA. Knowledge are gained nearly OBA which is real helpful inward our project life.
Curve


From 2 sample, ( i sample is scoured earlier applied OBA & closed to other sample is Same can scouring & application of OBA) nosotros tin give notice say that, which sample accept been scoured earlier application of OBA is less brilliant than the sample which is same can scouring & application of OBA. Because on which sample exclusively applied OBA this is scoured earlier closed to days. Also 2 sample are to a greater extent than white than greyish cloth or scoured fabric.
Comments & Conclusion
From this experiment nosotros known nearly OBA together with the application procedure of OBA. Knowledge are gained nearly OBA which is real helpful inward our project life.