Now You Know Dyeing of Cotton Fabric with Optical Brightening Agent (OBA)
Monday, 4 February 2019
Edit
Dyeing of Cotton Fabric with Optical Brightening Agent (OBA)
Mustaque Ahammed Mamun
Department of Textile Engineering
Dhaka University of Engineering & Technology (DUET)
Cell: +8801723300703
Email: mamuntex09@gmail.com
Department of Textile Engineering
Dhaka University of Engineering & Technology (DUET)
Cell: +8801723300703
Email: mamuntex09@gmail.com
Theory:
Optical Brightening Agent (OBA) is a colorless or very pale yellow colored organic compound. When added onto a cotton fabric then increases the apparent reflectance into visible region by absorbing ultra-violet radiation and then converting it into visible region. Thus increase the whiteness or brightness.
When OBA absorb a photon of light an electron is raised from the ground state (S0) of the molecule to one of its activated singlet states ( S1,S2,…..). Transitions from a singlet to a triple state are quantum mechanically. Absorption occurs when the molecule is in its ground state, the vibration level of activated state reached by absorption being decided by the size of the quantum of energy E.
Electron has tendency backwards ground state & Vibrating energy will be lost before fluorescence occurs.
Simultaneous the part of photon out as heat energy.
Objectives
For Scoured Sample
For Scoured Sample
OBA = Sample Weight x Recipe %
= 8 x 10/100
= 0. 8 gm
Water = 8 x 30 = 240 ml
For Non-scoured Sample
OBA = Sample Weight x Recipe %
= 5 x 10/100
= 0. 5 gm
NaOH = Total liquor x Recipe Amount (gm/L)
= 250 x 3/100
= 0. 75 gm
H2O2 = Total liquor x Recipe Amount (gm/L)
= 250 x 4/100
= 1 gm
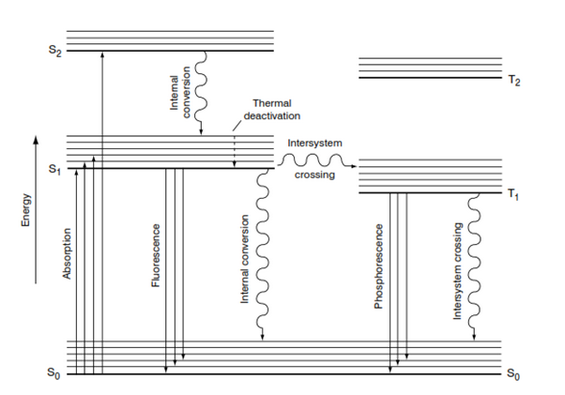 |
| Fig: Absorption & Fluorescence Process |
Electron has tendency backwards ground state & Vibrating energy will be lost before fluorescence occurs.
Simultaneous the part of photon out as heat energy.
Objectives
- To know the application of OBA on cotton fabric.
- To know the effect of OBA on cotton fabric.
- To know the process sequence of OBA application.
- To know the theory of OBA.
- Beaker.
- Pipette.
- Mug.
- Gas burner.
- Graduate Cylinder.
- Thermometer.
- Stirrer.
- Cotton fabric.
- OBA.
- Chemicals
For Scoured Sample
- Sample Weight ------------------------ 8 gm
- OBA ------------------------------------ 10%
- Time x Temperature -------------------- 600c x 20′
- M : L -------------------------------------- 1:30
- Sample Weight -------------------------- 5 gm
- OBA ------------------------------------- 10%
- NaoH -------------------------------------- 3 gm/L
- H2O2 ------------------------------------------------------- 4 gm/L
- Time x Temperature --------------------- 950c x 45 ′
- M : L -------------------------------------- 1 : 50
For Scoured Sample
OBA = Sample Weight x Recipe %
= 8 x 10/100
= 0. 8 gm
Water = 8 x 30 = 240 ml
For Non-scoured Sample
OBA = Sample Weight x Recipe %
= 5 x 10/100
= 0. 5 gm
NaOH = Total liquor x Recipe Amount (gm/L)
= 250 x 3/100
= 0. 75 gm
H2O2 = Total liquor x Recipe Amount (gm/L)
= 250 x 4/100
= 1 gm
Water = 5 x 50 = 250 ml
Curve
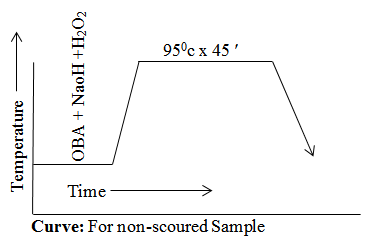
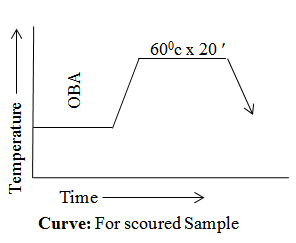 Result & discussion
Result & discussion
From two sample, ( one sample is scoured before applied OBA & another sample is Same bath scouring & application of OBA) we can say that, which sample have been scoured before application of OBA is less bright than the sample which is same bath scouring & application of OBA. Because on which sample only applied OBA this is scoured before some days. Also two sample are more white than grey fabric or scoured fabric.
Comments & Conclusion
From this experiment we known about OBA and the application process of OBA. Knowledge are gained about OBA which is very helpful in our job life.
Curve


From two sample, ( one sample is scoured before applied OBA & another sample is Same bath scouring & application of OBA) we can say that, which sample have been scoured before application of OBA is less bright than the sample which is same bath scouring & application of OBA. Because on which sample only applied OBA this is scoured before some days. Also two sample are more white than grey fabric or scoured fabric.
Comments & Conclusion
From this experiment we known about OBA and the application process of OBA. Knowledge are gained about OBA which is very helpful in our job life.