Yaa Basics Of Infrared Spectroscopy Inward Stuff
Tuesday, 18 December 2018
Edit
Basics of Infrared Spectroscopy inward Textile
Jaiparkash Kaushik
Department of Textile Technology
The Technological Institute of Textile in addition to Sciences,
Bhiwani, Republic of Republic of India
Email: jaiparkashkaushik@gmail.com
Department of Textile Technology
The Technological Institute of Textile in addition to Sciences,
Bhiwani, Republic of Republic of India
Email: jaiparkashkaushik@gmail.com
Introduction
Infrared Spectroscopy or IR Spectroscopy is used used to investigate vibrational construction of molecules or bonds inward textile material. They are equally good used to investigate the type of bonding betwixt the applied chemic in addition to the textile substrate. Hence, a basic noesis of IR Spectroscopy is important.
Spectroscopy is the report of the interaction of affair amongst the electromagnetic spectrum:
1. Electromagnetic radiations displays the properties of both particles in addition to waves,
2. The particle factor is called a photon,
3. The unloose energy (E) factor of a photon is proportional to the frequency . Where h is Planck’s constant in addition to n is the frequency inward Hertz (cycles per second),
E = hn
4. The term “photon” is implied to hateful a small, massless particle that contains a pocket-size wave-packet of EM radiation/light – nosotros volition purpose this terminology inward the course,
5. Because the speed of light, c, is constant, the frequency, n, (number of cycles of the moving ridge per second) tin consummate inward the same time, must endure inversely proportional to how long the oscillation is, or wavelength.
6. Amplitude, A, describes the moving ridge height, or forcefulness of the oscillation,
7. Because the atomic particles inward affair equally good present moving ridge in addition to particle properties (though contrary inward how much) EM radiations tin interact amongst affair inward 2 ways:
- Collision – particle-to-particle – unloose energy is lost equally estrus in addition to movement
- Coupling – the moving ridge holding of the radiations matches the moving ridge holding of the particle in addition to “couple” to the adjacent higher quantum mechanical unloose energy score
The IR Spectroscopic Process
1. The quantum mechanical unloose energy levels observed inward IR spectroscopy are those of molecular vibration,
2. We perceive this vibration equally heat,
3. When nosotros tell a covalent bond betwixt 2 atoms is of a surely length, nosotros are citing an average because the bond behaves equally if it were a vibrating bound connecting the 2 atoms,
4. For a uncomplicated diatomic molecule, this model is slow to visualize,
5. There are 2 types of bond vibration:
- Stretch – Vibration or oscillation along the trouble of the bond
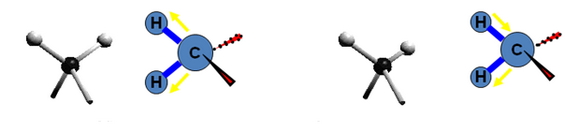 |
| Stretch |
- Bend – Vibration or oscillation non along the trouble of the bond
 |
| Bend |
6. As a covalent bond oscillates – due to the oscillation of the dipole of the molecule – a varying electromagnetic champaign is produced,
7. The greater the dipole instant modify through the vibration, the to a greater extent than intense the EM champaign that is generated,
8. When a moving ridge of infrared lite encounters this oscillating EM champaign generated past times the oscillating dipole of the same frequency, the 2 waves couple, in addition to IR lite is absorbed,
9. The coupled moving ridge forthwith vibrates amongst twice the amplitude.