Now You Know Assignment on Spandex Fiber
Friday, 8 March 2019
Edit
Comprehensive Study on Spandex Fiber

Md. Reazul Islam
B. Sc in Textile Engineering
Daffodil International University
Dhaka,Bangladesh.
Email: reaz.suzon@gmail.com
B. Sc in Textile Engineering
Daffodil International University
Dhaka,Bangladesh.
Email: reaz.suzon@gmail.com
Definition:
Spandex is a synthetic polymer. It is also called Elastane Fiber. Chemically, it is made up of a long-chain polyglycol combined with a short di-isocyanate, and contains at least 85% polyurethane. It is an elastomer, which means it can be stretched to a certain degree and it recoils when released. These fibers are superior to rubber because they are stronger, lighter, and more versatile. In fact, spandex fibers can be stretched to almost 500% of their length.
This unique elastic property of the spandex fibers is a direct result of the material's chemical composition. The fibers are made up of numerous polymer strands. These strands are composed of two types of segments: long, amorphous segments and short, rigid segments. In their natural state, the amorphous segments have a random molecular structure. They intermingle and make the fibers soft. Some of the rigid portions of the polymers bond with each other and give the fiber structure. When a force is applied to stretch the fibers, the bonds between the rigid sections are broken, and the amorphous segments straighten out. This makes the amorphous segments longer, thereby increasing the length of the fiber. When the fiber is stretched to its maximum length, the rigid segments again bond with each other. The amorphous segments remain in an elongated state. This makes the fiber stiffer and stronger. After the force is removed, the amorphous segments recoil and the fiber returns to its relaxed state. By using the elastic properties of spandex fibers, scientists can create fabrics that have desirable stretching and strength characteristics.
 |
| Spandex Yarns |
Spandex is used in a variety of different clothing types. Since it is lightweight and does not restrict movement, it is most often used in athletic wear. This includes such garments as swimsuits, bicycle pants, and exercise wear. The form-fitting properties of spandex makes it a good for use in under-garments. Hence, it is used in waist bands, support hose, bras, and briefs.
Types of spandex yarn
- Bare yarn.
- Covered yarn.
- Core spun yarn.
- Blend spun yarn.
The development of spandex was started during World War II. At this time, chemists took on the challenge of developing synthetic replacements for rubber. Two primary motivating factors prompted their research. First, the war effort required most of the available rubber for building equipment. Second, the price of rubber was unstable and it fluctuated frequently. Developing an alternative to rubber could solve both of these problems.
At first, their goal was to develop a durable elastic strand based on synthetic polymers. In 1940, the first polyurethane elastomers were produced. These polymers produced millable gums, which were an adequate alternative to rubber. Around the same time, scientists at Du Pont produced the first nylon polymers. These early nylon polymers were stiff and rigid, so efforts were begun to make them more elastic. When scientists found that other polyurethanes could be made into fine threads, they decided that these materials might be useful in making more stretchable nylons or in making lightweight garments.
The first spandex fibers were produced on an experimental level by one of the early pioneers in polymer chemistry, Farben fabriken Bayer. He earned a German patent for his synthesis in 1952. The final developments of the fibers were worked out independently by scientists at Du Pont and the U.S. Rubber Company. Du Pont used the brand name Lycra and began full scale manufacture in 1962. They are currently the world leader in the production of spandex fibers.
Molecular Structure:
Spandex is a polymer; its macromolecular structure is made up of repeating units (mars) denoted by the x and n next to the parentheses in the structure. Each Spandex fiber will differ somewhat in length and composition depending on the exact value of x and n.

 |
| Molecular Structure of spandex |
 |
| Microscopic view of spandex |
The most significant characteristic of spandex is its stretch ability. It can be stretched to a great length and then also recovers it’s near to original shape. It can, in fact, be stretched to almost 500% of its length. It is lightweight, soft, smooth, supple and more durable and has higher retractive ability than rubber. As such, when spandex is used for making any clothing, it gives the best fit and comfort and also prevents bagging and sagging of the garment. It is also heat-settable which means that it facilitates transforming puckered fabrics into flat fabrics, or flat fabrics into permanent rounded shapes. Spandex fibers or fabrics can be easily dyed and they also resist damage by body oils, perspiration, lotions or detergents. These fabrics are also abrasion resistant. When spandex is sewn, the needle causes little or no damage from "needle cutting" compared to the older types of elastic materials. The spandex fiber diameters range from 10 denier to 2500 denier and can be found in both, clear and opaque lusters.
Properties of Spandex:
Physical Properties of Spandex Fiber:
- Cross section- Spandex filaments are extruded usually from circular orifices, but the evaporation of solvent or the effects of drying may produce non-circular cross-sectional shapes. This may take various forms. In the multi-filament yarns, individual filaments are often fused together in places. The number of filaments in a yarn may be as few as 12 or as many as 50; the linear density of filaments ranges from 0.1 to 3 tex (g/km).
- Density: The density of spandex filaments ranges from 1.15 to 1.32 g/cc, the fibers lower density being based on polyesters.
- Moisture regain: The moisture of fibers from which the surface finish has been removed lies between 0.8 & 1.2%
- Length: It can be of any length. It may be used as filament or staple fiber.
- Color: It has white or nearly white color.
- Luster: It has usually dull luster.
- Strength: Low strength compared to most other synthetic fiber.
- Elasticity: Elastic properties are excellent. This is the outstanding characteristic of the fiber.
- Heat: The heat resistance varies considerably amongst the different degrades over 300 F.
- Flammability: It Burn slowly.
- Electrical conductivity: It has Low electrical conductivity.
- Breaking tenacity: 0.6 to 0.9grams/denier.
- Acid: Good resistance to most of acids unless exposure is over 24 hours.
- Alkalis: Good resistance to most of the alkalis, but some types of alkalis may damage the fiber.
- Organic solvents: Offer resistance to dry cleaning solvents.
- Bleaches: Can be degraded by sodium hypo-chloride. Chlorine bleach should not be used.
- Dyeing: A full range of colors is available. Some types are more difficult to dye than others
A variety of raw materials are used to produce stretchable spandex fibers. This includes pre-polymers which produce the backbone of the fiber, stabilizers which protect the integrity of the polymer, and colorants.
Two types of pre-polymers are reacted to produce the spandex fiber polymer back-bone. One is a flexible macro glycol while the other is a stiff di-isocyanate. The macro-glycol can be polyester, polyester, polycarbonate, polycaprolactone or some combination of these. These are long chain polymers, which have hydroxyl groups (-OH) on both ends. The important feature of these molecules is that they are long and flexible. This part of the spandex fiber is responsible for its stretching characteristic. The other pre-polymer used to produce spandex is a polymeric di-isocyanate. This is a shorter chain polymer, which has an isocyanate (-NCO) group on both ends. The principal characteristic of this molecule is its rigidity. In the fiber, this molecule provides strength.
 |
| Polyethylene |
In days before spandex, how did the corset contour the body effectively? In the eighteenth century, thick quilting and stout seams on the corset shaped the body when the garment was tightly laced. In the early nineteenth century, baleen, a bony but bendable substance from the mouth of the baleen whale, was sewn into seams of the corset (hence the term whalebone corsets); however the late 1800s corsets like this were stiffened with small, thin strips of steel covered with fabric. Such steel-clad corsets did not permit movement or comfort. By World War I, American women began separating parts of the corset into two garments—the girdle (waist and hip shaper) and bandeau (softer band used to support and shape the breasts).
Manufacturing Process:
Spandex fibers are manufactured in four different ways- melt extrusion, reaction spinning, solution dry spinning, and solution wet spinning. The initial step in all these methods is that of reacting monomers to produce a pre-polymer. Pre-polymer is then reacted further, in a variety of ways, and drawn out to produce a long fiber. The most commonly used method is the solution dry spinning that produces over 90% of the world's spandex fibers.
- First of all pre-polymer is produced by mixing a macro glycol with a di-isocyanate monomer. They are mixed in a reaction vessel and need the perfect conditions so that they may react to form a pre-polymer. Ratio of the component materials is responsible for giving different characteristics to the fibers. As such the ratio is strictly controlled. The ideal ratio of glycol to di-isocyanate may be 1:2.
- In the chain extension reaction, the pre-polymer is reacted with an equal amount of di-amine. It results in a solution which is diluted with a solvent to produce the spinning solution. The solvent makes the solution thinner which can be easily handled. It can then be pumped into the fiber production cell.
- In the fiber production cell, the polymer solution is pumped through a metal plate, called a spinneret, which has small holes throughout its structure. The solution gets aligned in strands of liquid polymer. The strands passing through the cell, are heated in the presence of a nitrogen and solvent gas. The liquid polymer gets chemically reacted and forms into solid strands.
- A specific amount of the solid strands are bundled together to produce the desired thickness with the help of a compressed air device that twists the fibers together. As such, it can be said that each fiber of spandex is made up of many smaller individual fibers that join one another due to the natural stickiness of their surface.
- The fibers are finally treated with textile finishing chemicals that can be magnesium stearate or other polymer such as poly(dimethyl-siloxane). These finishes prevent the fibers from sticking together and help in the process of textile manufacturing. Fibers are then transferred through a series of rollers onto a spool. The windup speed of the entire process depends on the thickness of the fibers that can be anywhere from 300-500 mi (482.7-804.5 km) per minute.
- The spools with fiber, are put into final packaging and shipped to textile manufacturers or any other customers. The fibers here, may be blended with other fibers such as nylon or cotton fiber to produce the fabric that is used for clothing purposes. These fabrics can also be dyed in order to give a desired color to them.
Groups (-OH) on the macro glycols react with the isocyanates. Each molecule gets added on to the end of another molecule, and a long chain polymer is formed. This is known as a step-growth or addition polymerization. To initiate this reaction, a catalyst such as di-azobicyclo octane must be used. Other low molecular weight amines are added to control the molecular weight of the fibers.
Spandex fibers are vulnerable to damage from a variety of sources including heat, light atmospheric contaminants, and chlorine. For this reason, stabilizers are added to protect the fibers. Antioxidants are one type of stabilizer.
Various antioxidants are added to the fibers, including monomeric and polymeric hindered phenols. To protect against light degradation, ultraviolet (UV) screeners such as hydroxybenzotriazoles are added. Compounds which inhibit fiber discoloration caused by atmospheric pollutants are another type of stabilizer added. These are typically compounds with tertiary amine functionality, which can interact with the oxides of nitrogen in air pollution. Since spandex is often used for swimwear, anti-mildew.
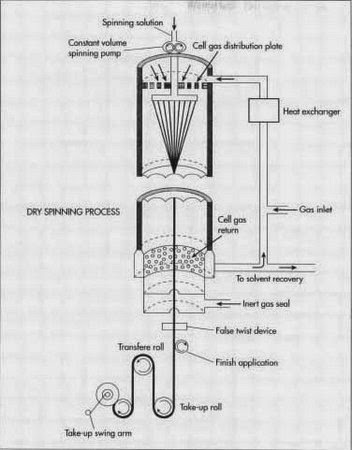 |
| Dry-spinning process. |
Additives must also be added. All of the stabilizers that are added to the spandex fibers are designed to be resistant to solvent exposure since this could have a damaging effect on the fiber.
When they are first produced, spandex fibers are white. Therefore, colorants are added to improve their aesthetic appearance. Dispersed and acid dyes are typically used. If the spandex fibers are interwoven with other fibers such as nylon or polyester, special dying methods are required.
 |
| Wet-spinning process |
Garments where comfort and fit are desired: Hosiery, swimsuits, aerobic/exercise wear, ski pants, golf jackets, disposable diaper, waist bands, bra straps and bra side panels.
 |
| Swimming dress |
 |
| Cycling Jersey |
The fiber discuss in this assignment which resemble rubber in that they have high extensibility and highly reactive forces which derive from their chemical nature. Natural polymers are usually better than we make ourselves and the development of spandex yarns may well spurs to rubber technologist to new achievement; in the past they have not had very much competition from other snap back fibers.
References:
- http://www.fibersource.com/f-tutor/spandex.htm
- http://en.wikipedia.org/wiki/Spandex
- http://www.teonline.com/knowledge-centre/spandex-fiber-production-process.html
- /search?q=mohair-or-angora-spandex-fiber