Now You Know Whitening Agent: Properties, Function, Mechanism and Usages (Part-7)
Saturday, 2 February 2019
Edit
Whitening Agent: Properties, Function, Mechanism and Usages (Part-7)
Authors: Md. Mosharaf Hossain
Kiriti Kingkar Mondal
Tawhidul Islam
Dept. of Textile Engineering
Primeasia University, Dhaka
Kiriti Kingkar Mondal
Tawhidul Islam
Dept. of Textile Engineering
Primeasia University, Dhaka
Previous Part
Graphical Representation of Whitening Process for Polyester Cotton Blended Fabric:
The whitening process for Polyester-Cotton blended fabric can be graphically presented as below : 
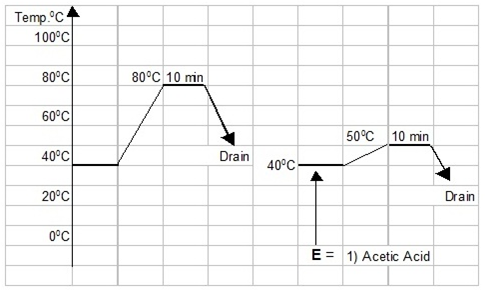 |
| Graphical view of whitening process for Polyester-Cotton blended fabric |
A simple OBA stripping technique given below can be adopted in jiggers very efficiently and you can strip off almost 98% of OBA.
Recipe and method of process flow given below may be used as new hint..
a) Wetting the fabric:
Soap - 1 gm/liter, Soda ash 1 gm/liter - treat the fabric at 70°C for 30 minutes.
b) Oxidative Bleaching:
Potassium Permanganate (KMnO4) - 0.5% wof at cold for 30 minutes - to the same bath add Hydrochloric acid (35% conc.), 3 times the quantity of KMnO4 continue run for another 30 minutes. Then raise the temperature to 50°C and run for 30 minutes.
Drain the bath - cold wash - 30 minutes.
c) Reductive Bleaching:
Run 10 minutes with Caustic soda 5 gms/liter and then add 5 gms/liter of Hydros (Sodium Hydro Sulphite) at room temperature and run for 10 minutes. Then slowly raise the temperature to 60°C. During this process the brown coloration you have got would be converted to light beige like shade. Now do one thorough cold wash.
4) Oxalic Acid Treatment:
Treat with 2 gms/liter of Oxalic acid at room temperature for 30 minutes or 2 ends followed by a cold wash.
5) Soda ash Neutralization:
Treat with 2 gms/liter of soda ash at room temperature for 30 minutes and again do a cold wash.
6) Acetic acid Neutralization:
Treat the fabric with 1 gm/liter of acetic acid for 10 minutes at cold. Check the pH and let it be 6.
Now you will wonder to see thorough removal of OBA.
Strategies for Use
Some of the critical factors in FWA use include (a) retention, (b) quenching, (c) competition with other UV-absorbers, and (d) metamerism. The strategies for adding and retaining FWAs to the wet end of a paper machine are very similar to those used with direct dyes. For instance, FWA retention can be increased by sequential addition of alum to the pulp stream, either before or after the whitener. But there is a key difference; highly charged cationic polyelectrolytes easily can destroy the fluorescent character of the molecule. The effect is called "quenching." (The effect is related, but much more problematic, than the tendency of the highly cationic polymers to modify the hue of certain direct dyes.) Both lignin and titanium dioxide are potent absorbers of ultraviolet light. For this reason, internal addition of FWAs to high-yield furnish or to furnish that contains TiO2 is likely to be ineffective. This is one of the reasons why it is common for papermakers to add all or most of the FWA at the size press. The idea is that light first encounters material nearer to the surface of the paper, and this is where much of the size-press formulation ends up. Metamerism refers to the phenomenon that two objects may appear to have identical color when viewed under a certain type of illumination, but the same objects might not match under a different lighting. This is a very common occurrence in the case of samples that contain different levels of activity of fluorescent whitening agents.
Common uses
Brighteners are commonly added to laundry detergents to replace whitening agents removed during washing and to make the clothes appear cleaner. Optical brighteners have replaced bluing which was formerly used to produce the same effect. Some brighteners can cause allergic reactions when in contact with skin, depending on the individual.
Brighteners are used in many papers, especially high brightness papers, resulting in their strongly fluorescent appearance under UV illumination. Paper brightness is typically measured at 457 nm, well within the fluorescent activity range of brighteners. Paper used for banknotes does not contain optical brighteners, so a common method for detecting counterfeit notes is to check for fluorescence.
Optical brighteners have also found use in cosmetics. One application is to formulas for washing and conditioning grey or blonde hair, where the brightener can not only increase the luminance and sparkle of the hair, but can also correct dull, yellowish discoloration without darkening the hair. Some advanced face and eye powders contain optical brightener microspheres that brighten shadowed or dark areas of the skin, such as "tired eyes".
A side effect of textile optical whitening is to make the treated fabrics more visible with Night Vision Devices than non-treated ones. This may or may not be desirable for military or other applications. Optically brightened paper is often not useful in exacting photographic or art applications, since the whiteness decreases with time.